Topics 9 & 19
Including International-mindedness, TOK, and 'Utilization' in Redox processes
International-mindedness
![]()
![]() 1. Aluminium production
1. Aluminium production
 The Hall-Hérault process for the production of aluminium by the electrolysis of alumina dissolved in molten cryolite was discovered independently in the 1880s by the American Charles Hall and the Frenchman Paul Hérault. Before then the price of aluminium had been prohibitively expensive as it had to be obtained by the chemical (rather than electrolytic) reduction of aluminium oxide. Even modern plants still provide a good example of the International-mindedness as the plants need to be sited where there is a plentiful supply of energy as the process is so energy intensive. A few years ago I was running a workshop in Bahrain and we had a field trip to the local aluminium plant. This uses oil is as the energy source (and in fact the ‘left over’ energy is used to power the whole of the rest of Bahrain). There is no aluminium ore in Bahrain so the bauxite is shipped in mainly from Australia and the aluminium product is then shipped all over the world. Another aluminium plant I visited recently near Reykjavik in Iceland uses geothermal energy as its energy source and again relies on shipping for the ore and its products.
The Hall-Hérault process for the production of aluminium by the electrolysis of alumina dissolved in molten cryolite was discovered independently in the 1880s by the American Charles Hall and the Frenchman Paul Hérault. Before then the price of aluminium had been prohibitively expensive as it had to be obtained by the chemical (rather than electrolytic) reduction of aluminium oxide. Even modern plants still provide a good example of the International-mindedness as the plants need to be sited where there is a plentiful supply of energy as the process is so energy intensive. A few years ago I was running a workshop in Bahrain and we had a field trip to the local aluminium plant. This uses oil is as the energy source (and in fact the ‘left over’ energy is used to power the whole of the rest of Bahrain). There is no aluminium ore in Bahrain so the bauxite is shipped in mainly from Australia and the aluminium product is then shipped all over the world. Another aluminium plant I visited recently near Reykjavik in Iceland uses geothermal energy as its energy source and again relies on shipping for the ore and its products.
![]()
![]() 2. International Gold Standard
2. International Gold Standard

The use of gold as the ultimate international monetary unit officially came to an end after the Second World War with the Bretton Woods Agreements. However even now people worldwide fall back on gold as a secure haven and hedge against inflation in times of uncertainty and the price of gold has fluctuated considerably during the recent global ‘Credit crunch’. There are several reasons why the Gold Standard has historically been the basis of an international monetary system in which the standard economic unit of account is a fixed weight of gold. These include rarity, durability, divisibility and ease of identification. However it is the inertness of gold that is key.
Au+(aq) + e− ⇌ Au(s) EAu3+(aq) + 3e− ⇌ Au(s) E
Gold has a very high positive standard electrode potential and can be transported and kept intact in air with no chemical changes occurring to it.
Theory of Knowledge
There are a few references relating redox processes to TOK given in the IB Chemistry Subject Guide for Topics 9 & 19. Sub-topic 9.1 Oxidation & reduction looks at language and questions the gains and losses caused by replacing older names. You can read more about this on the page on IUPAC on the TOK section of this website. Sub-topic 9.1 also asks whether artificial concepts such as oxidation numbers (or states) are useful. I have a particular issue with oxidation states as they are based on what is clearly a false assumption. Namely that to assign oxidation states to a covalent compound such as ammonia one has to assume that the compound is ionic.
![]()
![]() 1. In sub-topic 8.1 Theories of acids & bases the problem of giving one precise definition for an acid (or base) was highlighted. There is a similar problem with defining oxidation (and consequently reduction). In the past it was simple and very obvious. Oxidation is the addition of oxygen. One can hardly argue with that definition. The problem is that it has been extended. Initially it was extended to the loss of hydrogen but then to a definition involving electrons. Almost every IB student knows the mnemonic OILRIG (Oxidation Is Loss Reduction Is Gain) when applied to electrons. Past syllabuses have required students to define oxidation and reduction in terms of electron loss and gain and answers that state that oxidation is the loss of electrons have gained full marks in IB examinations. The current guide has changed this slightly so that it reads "Oxidation and reduction can be considered in terms of oxygen gain/hydrogen loss, electron transfer or change in oxidation number." Teachers usually introduce this by using an example such as burning magnesium in oxygen. The magnesium is obviously oxidized, it loses electrons and its oxidation state increases from 0 to +2 so it supports the definition. By default the oxygen is gaining electrons, hence it must be being reduced and this leads to the term redox reaction. However if teachers and students think more critically this does not prove that oxidation always involves a loss of electrons it just illustrates it. According to the Popper falsification theory one only needs to find one example that does not work to discredit the definition. In fact you do not have to look very far.
1. In sub-topic 8.1 Theories of acids & bases the problem of giving one precise definition for an acid (or base) was highlighted. There is a similar problem with defining oxidation (and consequently reduction). In the past it was simple and very obvious. Oxidation is the addition of oxygen. One can hardly argue with that definition. The problem is that it has been extended. Initially it was extended to the loss of hydrogen but then to a definition involving electrons. Almost every IB student knows the mnemonic OILRIG (Oxidation Is Loss Reduction Is Gain) when applied to electrons. Past syllabuses have required students to define oxidation and reduction in terms of electron loss and gain and answers that state that oxidation is the loss of electrons have gained full marks in IB examinations. The current guide has changed this slightly so that it reads "Oxidation and reduction can be considered in terms of oxygen gain/hydrogen loss, electron transfer or change in oxidation number." Teachers usually introduce this by using an example such as burning magnesium in oxygen. The magnesium is obviously oxidized, it loses electrons and its oxidation state increases from 0 to +2 so it supports the definition. By default the oxygen is gaining electrons, hence it must be being reduced and this leads to the term redox reaction. However if teachers and students think more critically this does not prove that oxidation always involves a loss of electrons it just illustrates it. According to the Popper falsification theory one only needs to find one example that does not work to discredit the definition. In fact you do not have to look very far. 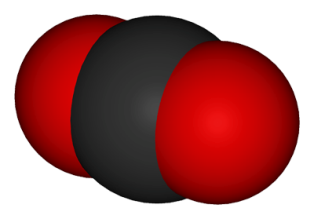 One of the most obvious examples of oxidation is the combustion of carbon in oxygen to form carbon dioxide. The carbon atoms in graphite contain four electrons each of their own and have a share in another four. In the product the carbon atoms still have four of their own electrons and have a share in another four (two each) from the two oxygen atoms. So carbon atoms have neither gained nor lost electrons and yet they have obviously been oxidised as they have gained oxygen. In other words oxidation does not always involve the loss of electrons. This provides a good example of how many teachers and students and text book authors (and IB examiners!) have stopped looking critically at what they are doing. I do not know who first coined the definition of oxidation in terms of electrons loss but I'm willing to bet either that he (or she) stated it the other way round that loss of electrons is oxidation or that he/she added in aqueous solution to the definition. Hydrogen gas reacting with oxygen gas is another example of a redox reaction where no electron transfer is involved but if the reaction is carried out in an electrolyte (i.e. in solution) then electrons are transferred and this forms the basis of the fuel cell. Similarly when using half-equations to balance redox reactions one uses H2O(l), H+(aq) or OH–(aq) to balance the equations, i.e. in aqueous solution.
One of the most obvious examples of oxidation is the combustion of carbon in oxygen to form carbon dioxide. The carbon atoms in graphite contain four electrons each of their own and have a share in another four. In the product the carbon atoms still have four of their own electrons and have a share in another four (two each) from the two oxygen atoms. So carbon atoms have neither gained nor lost electrons and yet they have obviously been oxidised as they have gained oxygen. In other words oxidation does not always involve the loss of electrons. This provides a good example of how many teachers and students and text book authors (and IB examiners!) have stopped looking critically at what they are doing. I do not know who first coined the definition of oxidation in terms of electrons loss but I'm willing to bet either that he (or she) stated it the other way round that loss of electrons is oxidation or that he/she added in aqueous solution to the definition. Hydrogen gas reacting with oxygen gas is another example of a redox reaction where no electron transfer is involved but if the reaction is carried out in an electrolyte (i.e. in solution) then electrons are transferred and this forms the basis of the fuel cell. Similarly when using half-equations to balance redox reactions one uses H2O(l), H+(aq) or OH–(aq) to balance the equations, i.e. in aqueous solution.
![]()
![]() 2. Naming and culture
2. Naming and culture
Hydrogen oxide, hydrogen oxide, every where,
And all the boards did shrink;
Hydrogen oxide, hydrogen oxide, every where,
Nor any drop to drink.
With apologies to Samuel Taylor Coleridge who wrote the Rime of the Ancient Mariner in 1798. Somehow it does not have the same feel as
Water, water, every where,
And all the boards did shrink;
Water, water, every where,
Nor any drop to drink.
.png) Chemists may find it convenient to rename substances in a logical way and it means that communication both within and between countries and cultures is easier. If you are not a chemist however then names like acetylsalicylic acid or (R)-4-(1-hydroxy-2-(methylamino)ethyl)benzene-1,2-diol are a lot less easy to remember or use than aspirin or epinephrine (adrenaline). It would be sad if everyone in the world called water hydrogen oxide or oxygen hydride. The Scandinavian aquavit would presumably have to be renamed hydrogenoxideavit and as for whisky which comes from usquebaugh the Gaelic for 'water of life' - well it just does not bear thinking about!
Chemists may find it convenient to rename substances in a logical way and it means that communication both within and between countries and cultures is easier. If you are not a chemist however then names like acetylsalicylic acid or (R)-4-(1-hydroxy-2-(methylamino)ethyl)benzene-1,2-diol are a lot less easy to remember or use than aspirin or epinephrine (adrenaline). It would be sad if everyone in the world called water hydrogen oxide or oxygen hydride. The Scandinavian aquavit would presumably have to be renamed hydrogenoxideavit and as for whisky which comes from usquebaugh the Gaelic for 'water of life' - well it just does not bear thinking about!
'Utilization'
![]()
![]() 1. Iron corrosion (rusting)
1. Iron corrosion (rusting)
Although the use of aluminium for construction is on the increase, iron is still the most widely used metal throughout the world. Iron is used for its strength, ease of working and cost.
 Even though there are many ways to prevent iron rusting, essentially by preventing the surface from coming into contact with air and water, the cost each year due to iron corrosion (rust) is massive. Rusting is a complex oxidation process that requires the presence of water and oxygen. The hydrated iron oxide formed is flaky so exposes a fresh surface of iron for the rusting to continue and also expands the volume of the iron which increase the likelihood of cracks appearing and forcing apart adjacent parts thus weakening the iron structure even further. It is difficult to get a precise figure for the cost of corrosion. Statements like ‘millions of dollars every year’ are not particularly helpful. One book “Corrosion basics: An introduction 2nd Edition” by P.R. Roberge claims that in the 1930s it was estimated that 2% of all the iron being used was lost annually through rust, and that since then the amount of iron in use has increased eighteen fold.
Even though there are many ways to prevent iron rusting, essentially by preventing the surface from coming into contact with air and water, the cost each year due to iron corrosion (rust) is massive. Rusting is a complex oxidation process that requires the presence of water and oxygen. The hydrated iron oxide formed is flaky so exposes a fresh surface of iron for the rusting to continue and also expands the volume of the iron which increase the likelihood of cracks appearing and forcing apart adjacent parts thus weakening the iron structure even further. It is difficult to get a precise figure for the cost of corrosion. Statements like ‘millions of dollars every year’ are not particularly helpful. One book “Corrosion basics: An introduction 2nd Edition” by P.R. Roberge claims that in the 1930s it was estimated that 2% of all the iron being used was lost annually through rust, and that since then the amount of iron in use has increased eighteen fold.
![]()
![]() 2. Battery technology
2. Battery technology
When covering voltaic cells in sub-topics 9.1 Electrochemical cells and 19.1 Electrochemical cells (AHL) it is worth commenting on how the recent developments in batteries have changed our social and working lives. Nowadays, when virtually every student in your class has their own mobile phone and spends much of each day surfing the Internet or communicating via social media on their phone, tablet or laptop it is hard to realize the changes that have taken place in society over the past decade or two. Much of this is due to the development of batteries. We now place much reliance on secondary (rechargeable) dry cell batteries such as Nickel metal hydride (NiMH) and lithium ion (Li-ion) cells. Even so the basic principle remains the same – the conversion of chemical energy to electrical energy via redox reactions. Rechargeable batteries are covered more fully in the AHL sub topic C.6 Electrochemistry, rechargeable batteries & fuel cells in Option C.

 IB Docs (2) Team
IB Docs (2) Team 