Stereoisomerism
 20.3 Stereoisomerism (3 hours)
20.3 Stereoisomerism (3 hours)
Pause for thought
The IB now distinguishes between conformational isomers and configurational isomers. However as conformational isomers exist in dynamic equilibrium their individual properties cannot easily be studied so this sub-topic is really only concerned with configurational isomers. These were formally sub-divided into geometric and optical isomers but the use of the term geometric isomers is no longer recommended.
The E/Z system has been on the syllabi of some other pre-university level courses for a while now. Even so, it may be new chemistry to many IB teachers. It is actually quite easy to apply and depends on what are known as the Cahn-Ingold-Prelog (CIP) rules for determining the priority of the atoms or groups attached to the two carbon atoms of the double bond. Each of these two carbon atoms on either side of the double bond is considered separately. In simple terms the higher the atomic number of the attached atoms to each carbon atom the higher the priority. Consider 1,2-dichloroethene. Chlorine has a higher atomic number than hydrogen so has a higher priority. If the two atoms/groups lie on the same side the isomer is Z and if they lie on opposite sides they are E. In this simple case Z is the cis- form and E is the trans- form.
Cl has a higher priority than H on the left hand side and on the right hand side.
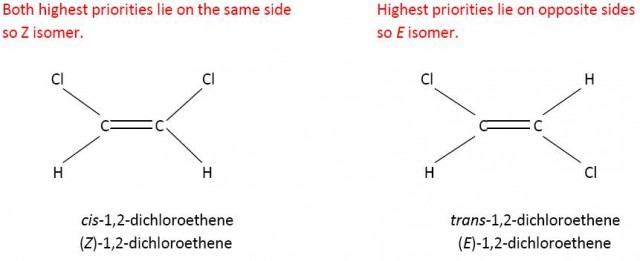
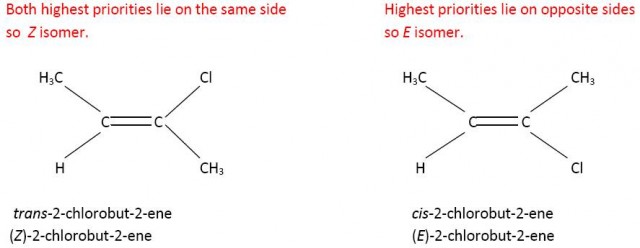
However Z does not always equate to cis-. If you consider 2-chloro-but-2-ene then the carbon atom of the methyl group has priority over the hydrogen atom on one side of the double bond but because chlorine has a higher atomic number than carbon the chlorine atom has priority over the methyl group on the other side of the double bond. Now the cis- isomer is the E isomer and the trans-isomer is the Z isomer.
CH3- has a higher priority than H on the left hand side and Cl has a higher priority than CH3- on the right hand side.
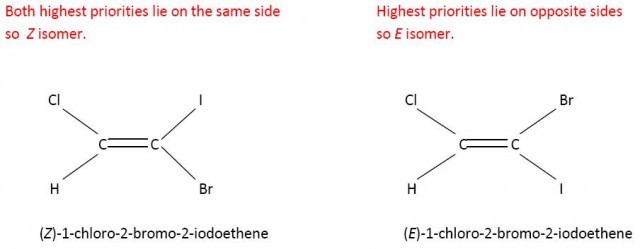
E and Z is more useful than cis- and trans- when the groups are all different. Consider 1-chloro-2-bromo-2-iodoethene. It is not obvious which would be the cis- and trans- forms. However using the Cahn-Ingold-Prelog rules the E and Z forms can easily be determined.
Cl has a higher priority than H on the left hand side and l has a higher priority than Br on the right hand side.
To help students it is worth them knowing the origin of Z and E. Both come from German words. Z is from zusammen (together) and E is from entgegen (opposite). However, if their German is not so good, one easy way to remember which is which is that E could stand for enemy, and enemies are on opposite sides.
You can read a very good explanation of this and what happens in the more complicated cases when different R groups are attached to the carbon atoms comprising the double bond on Jim Clark’s Chem Guide.
Nature of Science
The three-dimensional shape of an organic molecule is fundamental to both its structure and properties. Many of the molecules in the human body are chiral molecules that influence and determine how the body functions. This exemplifies the transdisciplinary nature of stereoisomerism.
Learning outcomesAfter studying this sub-topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesAccording to IUPAC the term geometric isomerism is now obsolete and the terms cis/trans isomers and E/Z isomers should be used. For the E/Z system, the group of highest Cahn–Ingold–Prelog priority attached to Optical isomers should be represented by using tapered wedges and dashes to show their bonds. International-mindednessDrugs and medicines produced by pharmaceutical companies around the world have been sold and administered as racemic mixtures instead of as the desired enantiomer with the associated therapeutic |
Teaching tipsIt is well worth each student, or pair of students, having access to a modelling set (e.g. Molymod) whilst teaching this topic. Virtual models can be useful but when they appear on a flat screen they are still 3-D being represented in two dimensions. Apart from knowing of its existence and why it occurs there is not very much that can be covered on conformational isomerism. It is probably worth getting students to make a 3-D model of butane and they can see how the two end methyl groups lie in different planes as the bond between the second and third carbon atoms is rotated. The isomers can be detected by infrared spectroscopy and the dynamics can be studied using NMR spectroscopy but this is beyond the syllabus. Give students plenty of practice at labelling different alkenes according to whether they are the E or Z form and emphasise how using E/Z is superior to cis/trans and the fact that the Z form is not always the cis form (or the E form is not always the trans form). Because there is a lot of human interest and it illustrates how profit can override ethical considerations it is worth students knowing about the thalidomide story to show the importance of enantiomers. Amino acids, 2-hydroxypropanoic acid and butan-2-ol are classic examples to use to illustrate chiral compounds but you can use many of the molecules given in Sections 33 and 37 of the data booklet as examples too. Make sure students can use the wedge and dotted line convention when making representations of enantiomers to show clearly mirror images. It can be difficult to know how much to do on diastereomers. It is probably worth showing how they can arise and stress that they can have different physical and chemical properties. There is a good article on this by UC Davis Chem Wiki. | Study guide
Page 94 - 96 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MC test: Stereoisomerism. For short-answer questions which can be set as an assignment for a test, homework or given for self study together with model answers see Stereoisomerism questions. Vocabulary listconformational isomer IM, TOK, Utilization etc.See separate page which covers all of Topics 10 & 20. Practical work
|
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A good video for clarifying the difference between enantiomers and diastereomers (diastereoisomers).
![]() Enantiomers and diastereoisomers
Enantiomers and diastereoisomers
2. E/Z isomerism explained. This video by Prof. Davis does exactly that.
3. The principles of polarimetry exemplified by HGTCChem.

 IB Docs (2) Team
IB Docs (2) Team 























