Aspirin & penicillin

 D.2 Aspirin & penicillin (5 hours)
D.2 Aspirin & penicillin (5 hours)
Pause for thought
It seems rather strange to me to have a sub-topic covering just aspirin and penicillin as they would appear to have little in common – even the ‘Understandings’ and ‘Applications and skills’ in the Guide list them under different headings. In previous versions of the programme, aspirin, together with paracetamol and ibuprofen, formed a sub-topic on mild analgesics and penicillin was part of a separate sub-topic on antibacterials.
However they do have one rather quirky thing in common and that is that their history of discovery in both cases is controversial.
Most sources state that aspirin was discovered in 1897 by Felix Hoffmann who was working for the Bayer Company in Germany at the time. The story goes that Hoffmann’s father suffered from arthritis and encouraged his son to develop an alternative treatment to sodium salicylate. Sodium salicylate is the sodium salt of salicylic acid obtained from the willow tree bark. It had been used for centuries as a painkiller and for the treatment for fever, but has several unpleasant side effects, including nausea, gastro-intestinal irritation, tinnitus and liver damage. This story first appeared only as a footnote in 1934 in a history of chemical engineering written by Albrecht Schmidt. Schmidt was an employee of IG Farbenindustrie, which had amalgamated with the Bayer Company in 1925. It seems likely however that another scientist working at Bayer in 1897 really deserves the credit. In 1949 Arthur Eichengrün (1867 - 1949) claimed that the work had been done under his direction and analysis of relevant archival and published material appears to support this claim. The reason it was not accepted at the time is that Eichengrün was a Jew and Jews were persecuted in Nazi Germany in the 1930s. In fact Eichengrün spent fourteen months in the Theresienstadt concentration camp.
Who discovered aspirin - Felix Hoffmann or Arthur Eichengrün?

Alexander Fleming is almost universally recognised as having made the serendipitous discovery of penicillin in 1928. Fleming was not able to stabilise the penicillin that he found and it was left to Florey and Chain during World War II to really develop it as an effective antibacterial. Fleming made his discovery while he was working at St Mary’s Hospital in London. One of the trustees of the hospital was Lord Beaverbrook who was a newspaper magnate and he used his power of the press to promote the story about Fleming. In fact the use of moulds to prevent infections has existed in folklore for a long time. In 1923 a Costa Rican, Clodomiro Picado Twight (1887 - 1944), working in Paris recorded the action of the fungal genus Penicillium sp. on the growth of bacteria, and his work was published in 1927, two years before Fleming published, but Clodomiro Twight has received little recognition outside of Costa Rica for his discovery.
Who discovered penicillin - Alexander Fleming or Clodomiro Picado Twight?

Nature of Science
The discovery of penicillin by Sir Alexander Fleming is one of the classic examples of serendipity in science - although there is some doubt that he was in fact the first person to discover it.
Many drugs have been isolated, identified and modified from natural sources. One example is derivatives of salicylic acid, obtained from the bark of the willow tree, which are used to relieve pain and fever.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesThe structures of aspirin and penicillin are provided in Section 37 of the data booklet. The ability of acidic (carboxylic) and basic (amino) International-mindednessAspirin is used worldwide and has several different uses. The discovery and use of antibacterials has changed the way that disease is treated throughout the world and contributed to an increase in life-expectancy. |
Teaching tipsThis is a relatively straightforward topic to teach although rather factual. Aspirin lends itself well to practical work e.g. Analysis of aspirin tablets and Preparation & purification of aspirin. In fact because the synthesis of aspirin is covered in the 'Understandings' it is well worth teaching it through the practical. The melting point and infrared spectrum of aspirin are also provided in this practical sheet. It is worth getting students to research for themselves the uses and problems (side effects) of aspirin and also how it works and perhaps get one of them to make a presentation. Explain that the solubility of aspirin in water can be increased considerably by making the sodium (or calcium) salt by reacting the carboxylic acid group with a base - this aids bioavailability. In the acid environment of the stomach the salt will revert back to its undissociated form. Many other drugs contain an amino group and these can be made water-soluble by reacting with an acid to form the corresponding salt, e.g. morphine hydrochloride. Students will be interested in antibiotics and the story of the discovery of penicillin is well worth telling. It is usually attributed to Alexander Fleming and the concept of serendipity is important here - use Pasteur's quote that 'Chance favours the prepared mind'. It is worth mentioning that the antibacterial properties of mould have been known for a long time and perhaps also challenge perceived wisdom by mentioning the earlier publication on penicillin by Clodomiro Twight (see 'Pause for thought' above) Equally fascinating is the development of penicillins by Florey and Chain during World War II. It is also worth talking about 'super bugs' such as MRSA and the problems of overprescribing and putting antibiotics in animal feedstock. | Study guidePages 155 &156 QuestionsFor ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Aspirin & penicillin. For short-answer questions see Aspirin & penicillin questions together with the worked answers on a separate page Aspirin & penicillin answers. Vocabulary list:analgesic Practical work
|
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. An informative article on taking aspirin for heart conditions and the risks associated with it has been produced by Patient UK. Another article also by Patient UK gives useful information on how mild painkillers work.
2. A useful podcast from the Royal Society of Chemistry on
3. A short video students will enjoy by Richard Thornley on the synergistic effects of taking alcohol with aspirin.
4. A good short video on how aspirin (and ibuprofen) prevent pain by TEDEd.
5. One of the first films on the discovery of penicillin was produced by the Wellcome Foundation in 1964. Rather patriotic (in British terms!) and very dated but worth showing.
6. A fairly simple description of how penicillins work by Tim Newman at about the right level for Standard Level students can be found in Medical News Today.
7. An interesting paper describing work done at Warwick University to understand how the penicillin resistant bacterium streptococcus pneumoniae is able to build up its immunity to penicillin.
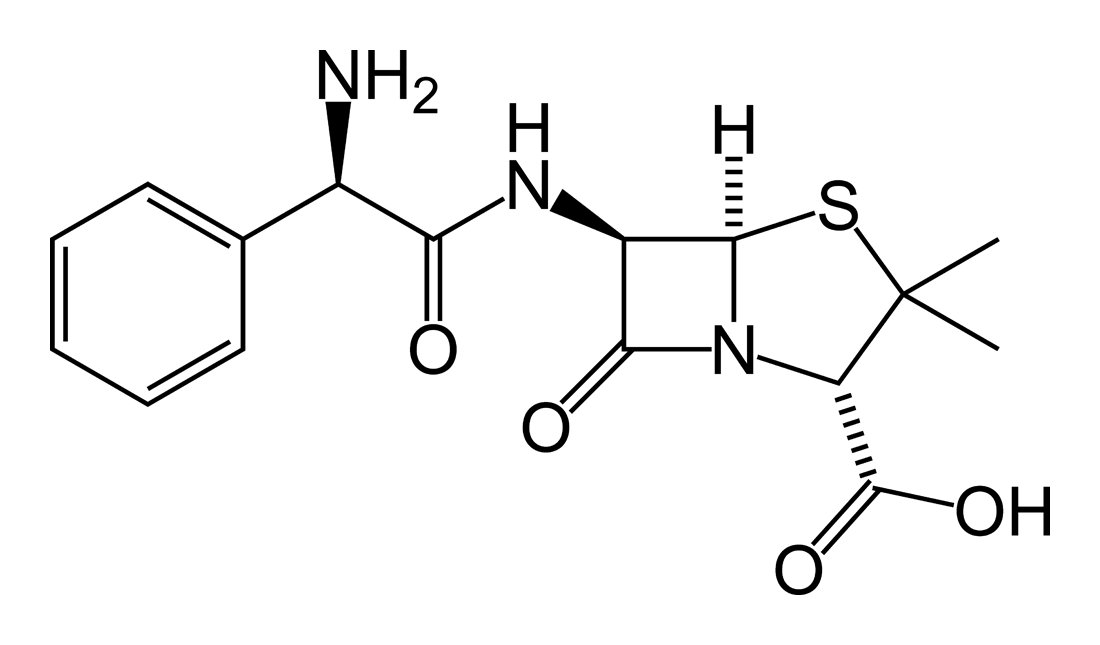
8. The structure of ampicillin ( a general purpose antibiotic) and two ampicillin 500 mg capsules.

 IB Docs (2) Team
IB Docs (2) Team 

























