Polymers answers

 Answers to questions on Polymers
Answers to questions on Polymers
Answers to Polymers questions.
1. (a) Because the reaction is an addition reaction there are no other products formed so all the reactants are converted into the desired product.
(b) Mass of 1000 H2N(CH2)6 NH2 = 1000 x 116.04 = 116040 g
Mass of 1000 ClOC(CH2)4COCl = 1000 x 183.04 = 183040 g
Total mass of reactants = 299080 g
Mass of H−[HN(CH2)6NHCO(CH2)4CO]1000−Cl = 299080 – 1999 x M(HCl) = 299080 – 72864 = 226216 g
Only 90% yield so mass of desired product = 0.9 x 226216 = 203594 g
Atom economy = (203594 ÷ 299080) x 100 = 68.1%
2. (a) 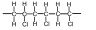
(b) During the mIB Docs (2) Teamfacture, while the PVC is thermosoftened, a plasticizer (such as a phthalate ester) is added.
(c) As the polymer cools, the plasticizer prevents the chains from forming such strong attractions (by forming ionic interactions between the plasticizer and the chains), which makes the polymer more flexible.
3. (a) Behaves like rubber by possessing elasticity and viscosity.
(b) Isotactic – methyl groups orientate in same direction.

Atactic - methyl groups randomly orientated.

Download the Answers to Polymers questions ![]()

 IB Docs (2) Team
IB Docs (2) Team