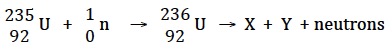Nuclear fusion & nuclear fission reactions

 C.3 Nuclear fusion & nuclear fission reactions (4 hours)
C.3 Nuclear fusion & nuclear fission reactions (4 hours)
Pause for thought

The moon - a source of helium-3?
Man first walked on the moon on 20 July 1969 and last set foot on the moon on 14 December 1972. Recently there has been increased interest in returning to the moon and one of the reasons for this is the potential to mine helium-3 as a future source of energy back on Earth.
The first hydrogen bomb tested in 1952 involved the reaction between deuterium and tritium (known as “D-T fusion”).
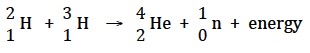
The syllabus states that, “Fusion reactions are a promising energy source as the fuel is inexpensive and abundant, and no radioactive waste is produced.” About one in every six thousand naturally occurring hydrogen atoms is deuterium so deuterium can be obtained from water. Tritium has to be made by reacting neutrons with lithium-6, but unlike deuterium it is radioactive.
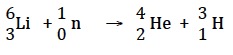
Another reaction that has been used to produce fusion energy is the reaction between two deuterium atoms (“D-D fusion”).
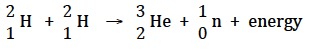
This has the advantage that the fuel is not radioactive but like the D-T fusion it produces energetic neutrons. These make the components of the reactor radioactive so radioactive waste is produced.
A much more promising source of future fusion energy is the reaction of helium-3 with itself. Helium-3 is not radioactive and since no neutrons are produced there is no radioactive waste either.
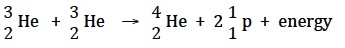
This reaction has been demonstrated to be feasible and really would be a clean fuel. The problem is that there although helium-3 does occur naturally on Earth as a decay product it is only in very small quantities and it has to be mIB Docs (2) Teamfactured from neutron bombardment. However it is much more prevalent on the moon and it has been estimated that just 25 tonnes of helium-3 could provide enough energy to satisfy all the energy requirements of the US for one year. Hence the renewed interest in lunar exploration.
Nature of Science
If the widespread use of nuclear fission can be used for energy production in the future, it would lead to a large reduction in greenhouse gas emissions.
The process taking place in the atomic bomb is nuclear fission whereas the process taking place in the hydrogen bomb is nuclear fusion.
Learning outcomesAfter studying this topic students should be able to: Understand: Nuclear fusion
Nuclear fission
Apply their knowledge to: Nuclear fusion
Nuclear fission
| Clarification notesStudents are not expected to learn specific fission reactions or how nuclear power plants work. Safety and risk issues that should be covered include: health issues, problems associated with nuclear waste and core meltdown, and the possibility that nuclear fuels may be used in nuclear weapons. Equations for nuclear decay and for calculating the half-life are given in Section 1 of the data booklet. International-mindednessThe International Atomic Energy Agency (IAEA). monitors the use of nuclear energy internationally Research into high energy particle physics involves international collaboration (there are accelerator facilities at CERN, DESY, SLAC, Fermi lab and Brookhaven). The results are disseminated and shared by scientists in many countries. The International Thermonuclear Experimental Reactor (ITER) project is a collaboration between many countries and aims to demonstrate that fusion is an energy source of the future. |
Teaching tipsIt is probably best to start this topic by looking at how the nuclear binding energy varies according to mass number. Although this is covered more fully in the AHL sub-topic C.7 students can see that the lighter elements below iron will tend to fuse to form heavier nuclei and the heavier elements above iron will tend to undergo fission to produce two or more lighter nuclei. Mass defect is also not covered until C.7 but students need some idea of the concept in order to see why nuclear fusion and fission are such powerful sources of energy. Isotopes have been covered in Topic 2: The nuclear atom but this will be the first time that students have come across nuclear equations. Give them plenty of practice at balancing these once they know the reactants and products and also deducing and balancing the products (or reactants) if they know the type of reaction taking place. In Topic 2: Electron configuration students will also have learned that each element has a unique emission spectra. You can revise this and build upon it to explain how the lines in the absorption spectra of stars can be used to identify the presence of specific elements. When introducing half-life I give them examples that they can solve by inspection first (i.e. using integer multiples of the half-life) so that they understand how it only takes a small number of half-lives before the percentage of the initial radioactive isotope is considerably reduced. Then introduce them to the equations. Three relevant equations are actually given in Section 1 of the data booklet but if they know t½ = 0.693/ λ and N = Noe−λt and how to use them they should be able to solve any half-life problems they come across. Discuss low-level and high-level nuclear waste with examples and how the waste can be dealt with. Safety at nuclear installations takes many forms. It may well be worth getting students to do their own research and discuss these amongst themselves. They may be surprised to find that in even some of the worst accidents, e.g. Chernobyl (1986) and Fukushima Daiichi (2011), the number of deaths reported was much lower than the number of deaths due to coal mining accidents every year. | Study guide
Pages 144 & 145 QuestionsFor ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Nuclear fusion & nuclear fission reactions. For short-answer questions see Nuclear fusion & nuclear fission questions together with the worked answers on a separate page Nuclear fusion & fission answers. Vocabulary list:fusion |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A simple but useful description of nuclear fission and how it can be controlled in a nuclear power plant by Fuse School- Global Education.
2. A more detailed account of nuclear energy which includes the importance of binding energy from The Science Channel (although you may need to explain about electron volts as Mev is used as the unit for energy).
3. A quick rundown on how to deal with nuclear waste by D News.

 IB Docs (2) Team
IB Docs (2) Team