Topics 11 & 21
Including International-mindedness, TOK, and 'Utilization' in Measurement, data processing & analysis
International-mindedness
![]()
![]() What are the 'true' values of units?
What are the 'true' values of units?
 The units that chemists use are international. A mole is the same worldwide. The SI unit of volume is a cubic metre although in practice a more useful unit is a cubic decimetre, dm3 which is called a litre in many countries. In the syllabus, and in examination questions, the IB uses dm3 and also cm3 but students who use litre, l, and millilitre, ml, respectively are not penalised as these are perfectly correct ways of expressing units of volume in many countries. How accurately are these units known and what is their ‘true’ value? For a metre the ‘true’ value was originally defined in France in 1793. It was further defined in 1889 as the distance between two lines marked on a bar composed of an alloy of platinum and iridium (90% Pt and 10% Ir) and this bar is still kept under specified conditions in the Bureau International des Poids et Mesures in Sevres, France (see image).
The units that chemists use are international. A mole is the same worldwide. The SI unit of volume is a cubic metre although in practice a more useful unit is a cubic decimetre, dm3 which is called a litre in many countries. In the syllabus, and in examination questions, the IB uses dm3 and also cm3 but students who use litre, l, and millilitre, ml, respectively are not penalised as these are perfectly correct ways of expressing units of volume in many countries. How accurately are these units known and what is their ‘true’ value? For a metre the ‘true’ value was originally defined in France in 1793. It was further defined in 1889 as the distance between two lines marked on a bar composed of an alloy of platinum and iridium (90% Pt and 10% Ir) and this bar is still kept under specified conditions in the Bureau International des Poids et Mesures in Sevres, France (see image).
As measurements became more precise and accurate this standard for the metre was insufficient. In 1960 it was redefined as equal to 1,650,763.73 wavelengths of the orange-red emission line in the electromagnetic spectrum of 86Kr in a vacuum. Since 1983 a metre has been further defined as "the length of the path travelled by light in vacuum during a time interval of 1/299,792,458 of a second." This reduces uncertainty even further but now, of course, this definition also depends on the accurate definition of a second!
Similar redefining has taken place with the definition of a mole and the atomic mass of an element. Because of advantages in analytical chemistry atomic masses can now be measured much more precisely than in the past. This has led to a recent change in the atomic mass of eleven elements. For these elements, which occur naturally as more than one isotope, the atomic mass is now given as lying between two values. For example, the range for hydrogen is now given as 1.007 84 to 1.008 11. For chemistry at IB level, where generally values to three significant figures (or for atomic mass two decimal places) are used, these changes in volume and atomic mass are irrelevant. However for precise work particularly when dealing with the chemistry of other galaxies where large distances and time are involved these changes can make a significant difference. Even with these precise values for a metre and atomic numbers it is still worth noting that none of them comes with the IB + or – uncertainty that our students are supposed to always provide with their experimentally determined results!
Theory of knowledge
The whole topic of measurement and uncertainty is inherently concerned with TOK since we could ask how can we be certain of anything if there is an uncertainty associated with all quantitative measurements upon which conclusions are based?
![]()
![]() 1. What is the accepted 'literature value'?
1. What is the accepted 'literature value'?
When writing their conclusion for their assessed individual scientific investigation students are encouraged to compare their result with the 'literature value' and hence deduce their percentage error and use this as the basis to evaluate the validity or otherwise of their experimental method. The literature value is often obtained from a data book. There are two comments to make here. The first is that different data books often quote different values. For example, consider the values given for the boiling point of cyclohexene from reputable data books and text books:
82.98 oC1
83.3 oC2
83 oC3
1. D.R. Lide (Ed.), Handbook of Chemistry & Physics, 88th. Ed., CRC Press, 2007
2. J.G. Stark & H.G.Wallace, Chemistry Data Book, 2nd. Ed., John Murray, 1989
3. R.T. Morrison & R.N. Boyd, Organic Chemistry, 6th. Ed., Prentice-Hall, 1992
Students of course will often go to the Internet to obtain the literature value. Wikepedia lists 82.98 oC, which is presumably taken from the CRC book. However the reputable website from the RSC, ChemSpider lists many values:
82 °C/mmHg (Tokyo Chemical Industry Ltd)
83-85 deg C (Alfa Aesar)
83-85 deg C / mmHg (Alfa Aesar)
181F (NIOSH)
80.7 C (Fisher MSDS)
Clearly there are many different values given (including a difference in the number of significant figures) so which is the true one upon which students should work out their percentage error? The second point is that although the IB insists on students giving the uncertainty associated with their calculated result this is obviously not how science works in reality since none of the literature values come with any associated uncertainty.
![]()
![]() 2. Heisenberg's Uncertainty Principle
2. Heisenberg's Uncertainty Principle
It is worth stressing to students that not only is there an uncertainty due to the apparatus or instrument used to take any measurement but there is an inherent uncertainty in the system itself. Any attempt to make a measurement actually affects what is being measured. The more accurately the position of a particle is known the less accurately its momentum is known and vice-versa. For very small particles such as electrons this effect is significant but it is even present, although to a much less significant extent, for particles with greater mass.
'Utilization'
The syllabus makes several references to utilizing spectroscopic techniques. For example magnetic resonance imaging (MRI) which gives a three-dimensional view of organs in the human body and asks why MRI is replacing computerized tomography (CT) scans for some applications but is used as a complementary technique for others. It also mentions the forensic applications of chromatography and spectroscopy and the use of analytical techniques for drug testing. Under topic 11 it mentions the problems with the incorrect measurement of the speed of neutrinos when scientists at CERN pronounced that the neutrinos were travelling faster than the speed of light and the crash of the Mars Climate Orbiter spacecraft (see below). Under graphing in 11.2 it includes using graphical data in areas such as population, finance and climate modelling. The statistical trends can lead to predictions and to the underpinning of government policies in areas such as health and education. Some particular examples of utilization are:
![]()
![]() 1. Global warming
1. Global warming
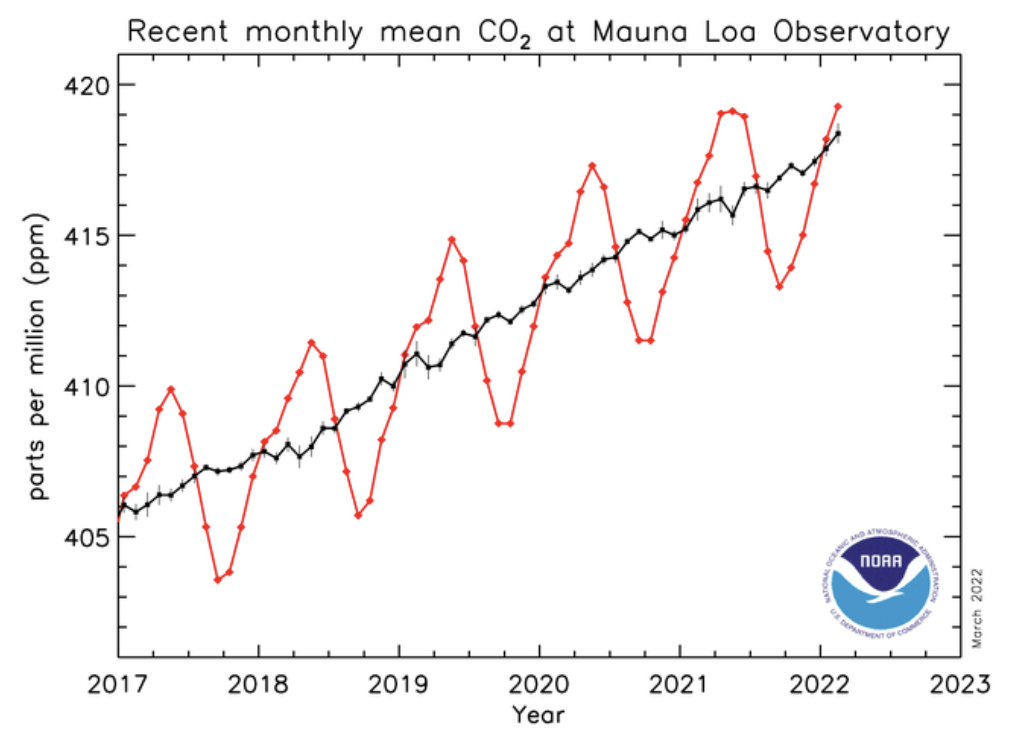 Is the current global warming man-made? It seems to be generally accepted by informed people now that the increase in the combustion of fossil fuels since the industrial revolution has led to an increase in the carbon dioxide concentration in the atmosphere. There has also been an increase in global warming due to the greenhouse effect. There is good scientific evidence from the Mauna Loa Observatory, in Hawaii that carbon dioxide levels have increased (and are still increasing - see graph on left) and that the average temperature of the Earth has increased during the past century but there is no certainty at all, just informed speculation, that this is all due to just human activities.
Is the current global warming man-made? It seems to be generally accepted by informed people now that the increase in the combustion of fossil fuels since the industrial revolution has led to an increase in the carbon dioxide concentration in the atmosphere. There has also been an increase in global warming due to the greenhouse effect. There is good scientific evidence from the Mauna Loa Observatory, in Hawaii that carbon dioxide levels have increased (and are still increasing - see graph on left) and that the average temperature of the Earth has increased during the past century but there is no certainty at all, just informed speculation, that this is all due to just human activities.
![]()
![]() 2. Food and drug research
2. Food and drug research
Often research is quoted in newspapers and online about how particular foods are good or bad for your health. For example, one study in 2013 published in the American Journal of Epidemiology, found that drinking coffee is associated with a reduced risk of oral cancer. This study was made from data obtained from 968,432 individuals and the uncertainty associated with the outcome is listed. Contrast this to some claims made by supposedly independent research into other foodstuffs and drugs. For example, a team of researchers led by Dr Robert McNamara at the University of Cincinnati, USA, reported in 2010 in the American Journal of Clinical Nutrition that boys aged 8-10 who take daily dosages of docosahexaenoic acid showed a big improvement in their ability to concentrate and in their performance doing tasks that involved attention. This research involves just 33 individuals who were not randomly selected and the research was paid for by the company that produces the fish oil containing the docosaheaxaenoic acid. How high is the uncertainty associated with such results? You can read more about this in my blog article.
![]()
![]() 3. Mars Climate Orbiter spacecraft
3. Mars Climate Orbiter spacecraft
In 1998 NASA launched a robotic space probe called the Mars Climate Orbiter which was intended to study the Martian climate. However nine months later communication with the spacecraft was lost as they tried to programme it to go into orbit around Mars. The spacecraft eventually disintegrated in the upper atmosphere of Mars as it was on the wrong trajectory which took it too close to the planet. The reason was because the computer software used on the ground was programmed in the non-SI units of pound-seconds whereas the onboard computers were operating in the different units of newton seconds.
![]()
![]() 4. Testing athletes for drugs
4. Testing athletes for drugs
 Testing for drugs is now an integral part of any major sporting event. Two of the most famous athletes who have been caught cheating are the 100 m sprinter Ben Johnson (see image on right) and Lance Armstrong, the seven times winner of the Tour de France cycle race.
Testing for drugs is now an integral part of any major sporting event. Two of the most famous athletes who have been caught cheating are the 100 m sprinter Ben Johnson (see image on right) and Lance Armstrong, the seven times winner of the Tour de France cycle race.
Both urine and blood samples from athletes (and race horses) are tested for a huge range of banned substances. Probably the main method of determining the presence and amount of a banned substance in the body is gas liquid chromatography linked to mass spectrometry (GLC-MS). The GLC separates the substances present in a urine sample according to their retention times and then they pass through a mass spectrometer where their spectra are compared with a database of known samples. Another technique that is sometimes used for specific substances is immuno-assay. All the techniques are now so sophisticated that they capable of detecting minute quantities of banned substances or their breakdown products.

 IB Docs (2) Team
IB Docs (2) Team