Periodic trends

 3.2 Periodic trends (5 hours)
3.2 Periodic trends (5 hours)
Pause for thought
1. The graph of first ionization energies against atomic number (see Topics 2 & 12) exemplifies periodicity in classic style. But periodicity is not always so clear cut when other properties of the elements are plotted against atomic number. For example, consider the graph for melting points.
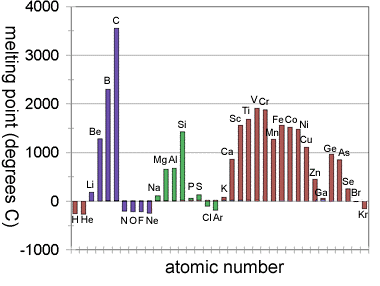
Graph of melting points plotted against atomic number for the first 36 elements.
There are some signs of periodicity. For example, carbon, silicon and germanium (group 14) follow a trend, but look at groups 2 and 13. Boron is much higher than beryllium (period 2), magnesium and aluminium are virtually the same (period 3) and gallium is lower than calcium (period 4). Get students to plot other graphs and see how obvious the concept of periodicity really is. It will make them appreciate the genius of Mendeleev even more.
2. A knowledge of the chemical properties of the elements and oxides of period 3 can have practical uses. Many motorcycles produced in the 1950s, such as this classic T100 Triumph Tiger mIB Docs (2) Teamfactured in 1951, had aluminium alloy engines.

1951 T100 Triumph Tiger motorcycle with an alloy engine
They were renowned for leaking oil and one of the ways some people tried to clean the engines to remove the grease and oil was with oven cleaner. Not a good idea. Some oven cleaners contain sodium hydroxide or potassium hydroxide. This reacts with the amphoteric aluminium oxide layer on the engine and then, in turn, with the exposed aluminium underneath to turn the motorcycle engine into sodium (or potassium aluminate) and potentially explosive hydrogen gas!
Nature of Science
Scientists are able to make accurate predictions about the physical and chemical properties of an element based on its position in the periodic table. By identifying and using patterns scientists are able to synthesize new substances based on the expected reactivity of elements.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesOnly examples of general trends across periods and down groups are required. International-mindednessBecause of industrialization, many products are produced that cause global problems when released into the environment. |
Teaching tipsTo help students gain a good picture of the physical trends I suggest you get them to use databases and simulations and try to first explain the trends themselves. It is not immediately obvious that as atoms of elements gain in mass across a period they get smaller in size. However if students understand that electrons are being placed in the same energy level which is being attracted closer to the nucleus as the nucleus gains more protons it then become obvious. It is worth including a brief discussion about what is meant by atomic radius and how it can be measured - after all you cannot precisely locate any electron so it cannot simply be the distance from the nucleus to the outermost electron! Ionic radii are slightly harder. Firstly because sometimes ionic radius refers to positive ions and sometimes to negative ions. Also the charge on the ion changes depending upon the group the element is in. Get them to deduce the number of protons and the electron configuration. The relevant ions will all have the electron configuration of a rare gas and when the number of protons is taken into account it should be obvious how the sizes change both down a group and across a period. Although the word as such is not specified on the syllabus it can be useful to introduce the students to the word 'isoelectronic' here. Melting points are harder to explain and in fact an explanation is not required. It depends both on the attractive forces between the atoms (for metals and non-metallic macromolecules such as diamond, graphite and silicon) or molecules (for other non-metals) and the type of packing. I do not cover electronegativities at this point. They do not make sense until you are covering bonding as they imply knowledge of covalent bonding. I include them when I am discussing whether compounds are likely to be bonded ionically or covalently when I teach Topic 4 : Chemical bonding & structure. When you do cover electronegativity stress that there are no units and Pauling's scale is only one way of giving them values. For the chemical properties it is well worth letting them loose on as many of the period 3 elements and their oxides as you can safely find. If you can demonstrate some of the more potentially dangerous reactions of the alkali metals and the halogens to them so much the better but if not show the videos below in 'other resources'. The redox reactions of the halogens with halide ions should be done practically by students but you may wish to leave these until you cover Topics 9: Redox processes so that they can fully understand them and write the half-equations. Make sure students can explain the trends of the oxides across the period. Stress that the nature of their oxides is one of the best chemical differences between metals and non-metals. You will need to give them the equations for the reactions of Na2O, MgO, P4O10 and the oxides of sulfur and nitrogen with water. Discuss the international problems caused by the release of oxides of sulfur and nitrogen - acid deposition is no respecter of national boundaries. | Study guide
Pages 17 - 19 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MC test: Periodic trends. For short-answer questions which can be set as an assignment for a test, homework or given for self study together with model answers see Periodic trends questions. Vocabulary listFirst ionization energy
|
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. The Interactive periodic table produced by Brain Adams for touchspin has already been mentioned in Topics 3 & 13. It is an alternative database to the RSC resource given above under 'suggestions for practical work'.
2. This might be an interesting video for you to look at and consider how you teach this topic. Do you think this is good or bad teaching? Some of the comments that students have written underneath are worth considering too.
3. The classic video clip from the Royal Society of Chemistry (or was it the Open University?) of the alkali metals reacting with water.
4. And now for the Brainiac version of the same reactions. Much more exciting and students love it but better for TOK than for genuine chemistry! Look out for the perfect let-out clause, "Only on Brainiac do you get that kind of science".
Brainiac alkali metals with water ![]()
5. A nice video to show from the Open University on the chemistry of the four halogens, fluorine, chlorine, bromine and iodine.

 IB Docs (2) Team
IB Docs (2) Team 





































