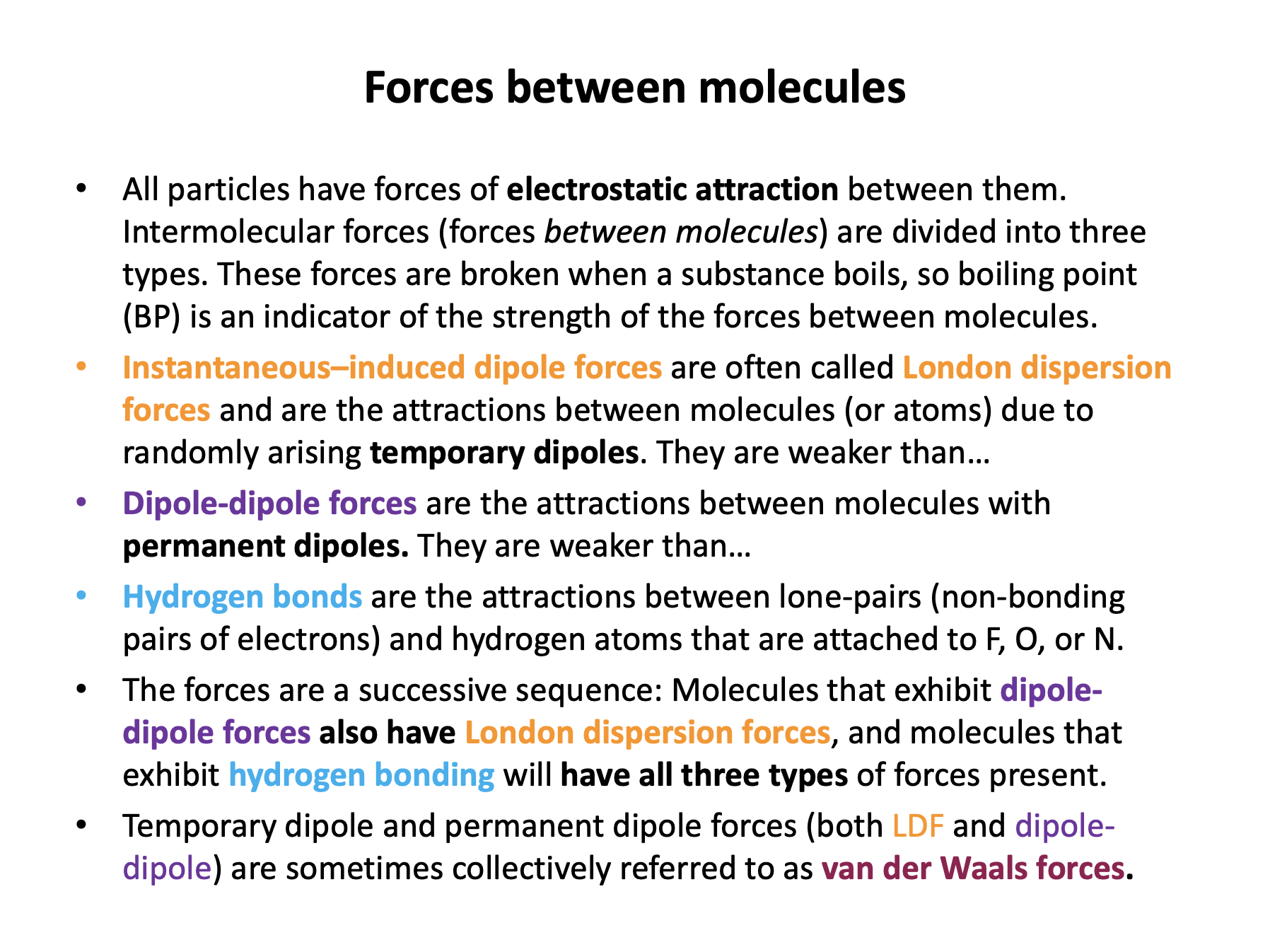
 Intermolecular forces are the electrostatic attractions between molecules. They tend to be weaker than ionic, covalent or metallic bonds. Intermolecular forces are divided into three types; London dispersion forces, dipole-dipole forces and hydrogen bonding. There is quite a lot of information in this section to learn, and vocabulary that must be used precisely!
Intermolecular forces are the electrostatic attractions between molecules. They tend to be weaker than ionic, covalent or metallic bonds. Intermolecular forces are divided into three types; London dispersion forces, dipole-dipole forces and hydrogen bonding. There is quite a lot of information in this section to learn, and vocabulary that must be used precisely!
Ensure you are confident using the terms below and learn the asterisked* definitions
a polar molecule, a non-polar molecule, London dispersion forces, dipole-dipole forces, hydrogen bonds, van der Waals forces, miscibility.
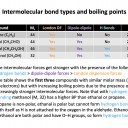
What is the accepted relative strengths of the three types of intermolecular force?
The accepted relative strengths are that hydrogen bonding is greater in strength than dipole-dipole forces that are in turn greater in strength than London dispersion forces (Hydrogen bonding > dipole-dipole forces > London dispersion forces).
Which of the following intermolecular forces are found between molecules of ethanol, C2H5OH?
Ethanol molecules exhibit London dispersion forces, dipole-dipole forces, and hydrogen bonding between the molecules.
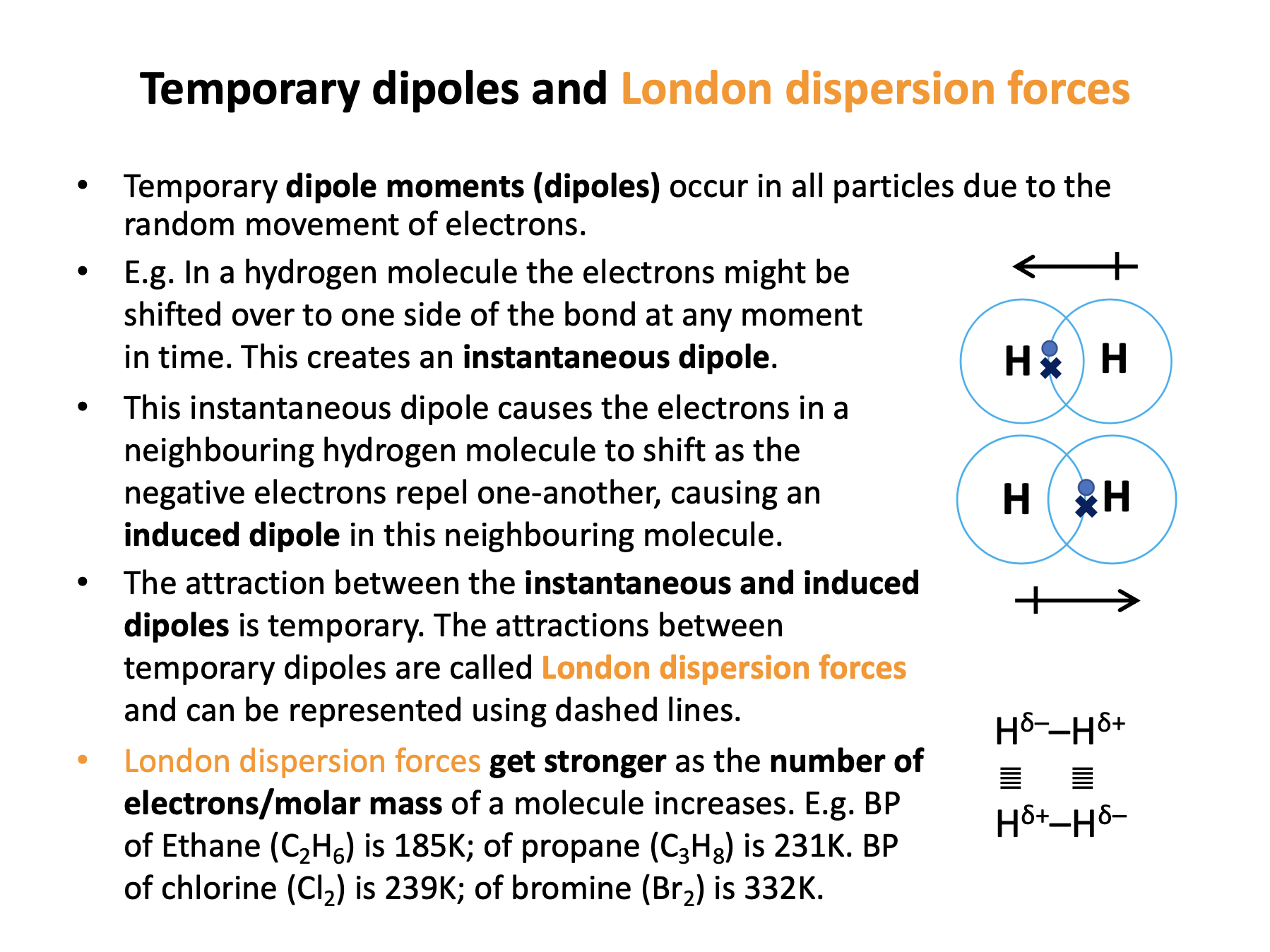
All molecules have London dispersion forces between them.
Molecules with permanent dipoles have dipole-dipole forces: Ethanol has a permanent dipole as it has polar bonds, C–O and O–H, and asymmetric geometry.
Molecules with a hydrogen atom attached to F, O or N possess hydrogen bonding between molecules: Ethanol has an O–H bond, so also has hydrogen bonding between molecules.
Which of the following intermolecular forces are found between molecules of propanal, CH3CH2CHO?
Propanal is an aldehyde; aldehyde molecules exhibit London dispersion forces and dipole-dipole forces, but do not have hydrogen bonding between the molecules.
All molecules have London dispersion forces between them.
Molecules with permanent dipoles have dipole-dipole forces: Propanal has a permanent dipole as it has polar bonds, C=O, and asymmetric geometry.
Molecules with a hydrogen atom attached to F, O or N possess hydrogen bonding between molecules: Propanal does not have an O–H bond (the bonds in the functional group are C=O and C–H) so does not have hydrogen bonding between molecules.
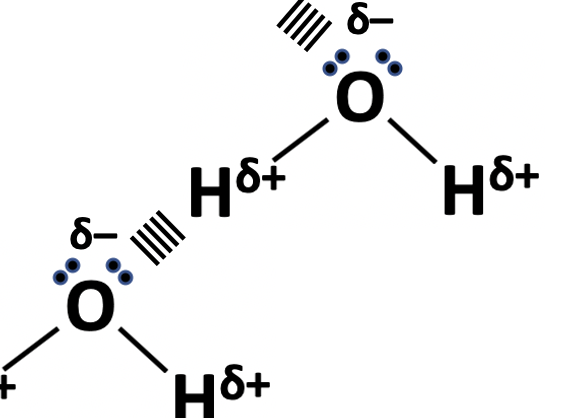
Which of the following should be included in the diagram when illustrating a hydrogen bond between water molecules?
1: The lone pairs on the oxygen atom.
2: The dipoles on the O–H bonds.
3: Dotted lines to represent the electrostatic attraction between two hydrogen atoms.
Diagram below:
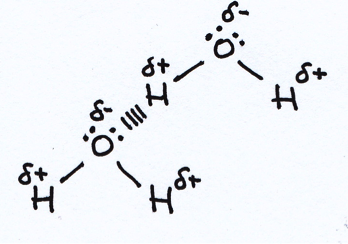
Note that the electrostatic attraction is between a lone pair on the oxygen atom and the δ+ hydrogen atom (not between two hydrogen atoms as in option 3). Thus lone pairs on the oxygen atom are included (option 1), as is the dipole on the O–H bonds (option 2).
The correct answer is 1 and 2 only.
Simple (molecular) covalent compounds do not conduct electricity. Water from a tap always conducts electricity when tested in a laboratory at room temperature. What is the best explanation for the conductivity of tap water?
Water is a simple molecule and when truly pure has very poor electrical conductivity.
Water can dissociate into H+ and OH– ions, but they are very few in number and do not provide good conductivity at room temperature.
Water is highly polar, but polar molecules alone cannot conduct electricity, and water does not have delocalised electrons.
It is the ability of water (being so polar) to dissolve ions that results in good electrical conductivity – the dissolved ions in the water can move and carry charge. Thus the correct answer is: Water is highly polar and dissolved ions in the water can move and carry charge.
Compound Y consists of molecules and is a colourless liquid at room temperature. It is highly volatile and immiscible with water. What are the likely intermolecular forces present in compound Y?
A high level of volatility (meaning evaporates easily) suggests very weak forces between molecules (only London dispersion forces). Immiscibility with water (not mixing with water; forming two separate layers) suggests that the compound is not polar, and certainly does not have hydrogen bonds.
Thus 'London dispersion forces only' is the correct answer.
Paper 1
Core (SL&HL): Bonding and Structure core (SL and HL) paper 1 questions
AHL (HL only): Bonding and Structure AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Bonding & Structure core (SL & HL) paper 2 questions
AHL (HL only): Bonding & Structure AHL (HL only) paper 2 questions
How much of Intermolecular forces have you understood?










 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn