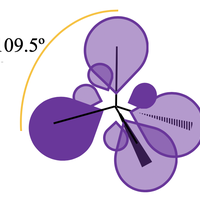
 It is really important that you are confident with your molecular shapes (covalent structure 4.3) and formation of sigma and pi bonds (further aspects of covalent bonding 14.1) before you try to get to grips with hybridization. Hybridization is a section of the course that is very challenging conceptually. The challenge is that hybridization is an abstract adjustment to an abstract model! However, the important thing to remember is that it is just a change to our model. We use VSEPR to explain the shapes of molecules through the Lewis (dot-cross) model of bonding. And we use hybridization to allow us to explain the shapes of molecules through the quantum mechanical model of the atom; to adjust the arrangement of the atomic orbitals in three-dimensional space. If, despite efforts, you are struggling to get your head around hybridization; the last slide of the revision cards has a useful rule-of-thumb that you can apply to help deal with exam questions.
It is really important that you are confident with your molecular shapes (covalent structure 4.3) and formation of sigma and pi bonds (further aspects of covalent bonding 14.1) before you try to get to grips with hybridization. Hybridization is a section of the course that is very challenging conceptually. The challenge is that hybridization is an abstract adjustment to an abstract model! However, the important thing to remember is that it is just a change to our model. We use VSEPR to explain the shapes of molecules through the Lewis (dot-cross) model of bonding. And we use hybridization to allow us to explain the shapes of molecules through the quantum mechanical model of the atom; to adjust the arrangement of the atomic orbitals in three-dimensional space. If, despite efforts, you are struggling to get your head around hybridization; the last slide of the revision cards has a useful rule-of-thumb that you can apply to help deal with exam questions.
Ensure you are confident using the terms below and learn the asterisked* definitions
hybridization*, sp3 hybridization, sp2 hybridization, sp hybridization
Which words are missing from the sentence below to give the best definition of hybridisation:
Hybridisation is the mixing of _______________ _______________ on the same atom, to produce hybrid orbitals.
'atomic orbitals' is the correct answer.
Hybridisation is a theory used to justify the shape of molecules when considering the combination of atomic orbitals to form sigma and pi bonds.
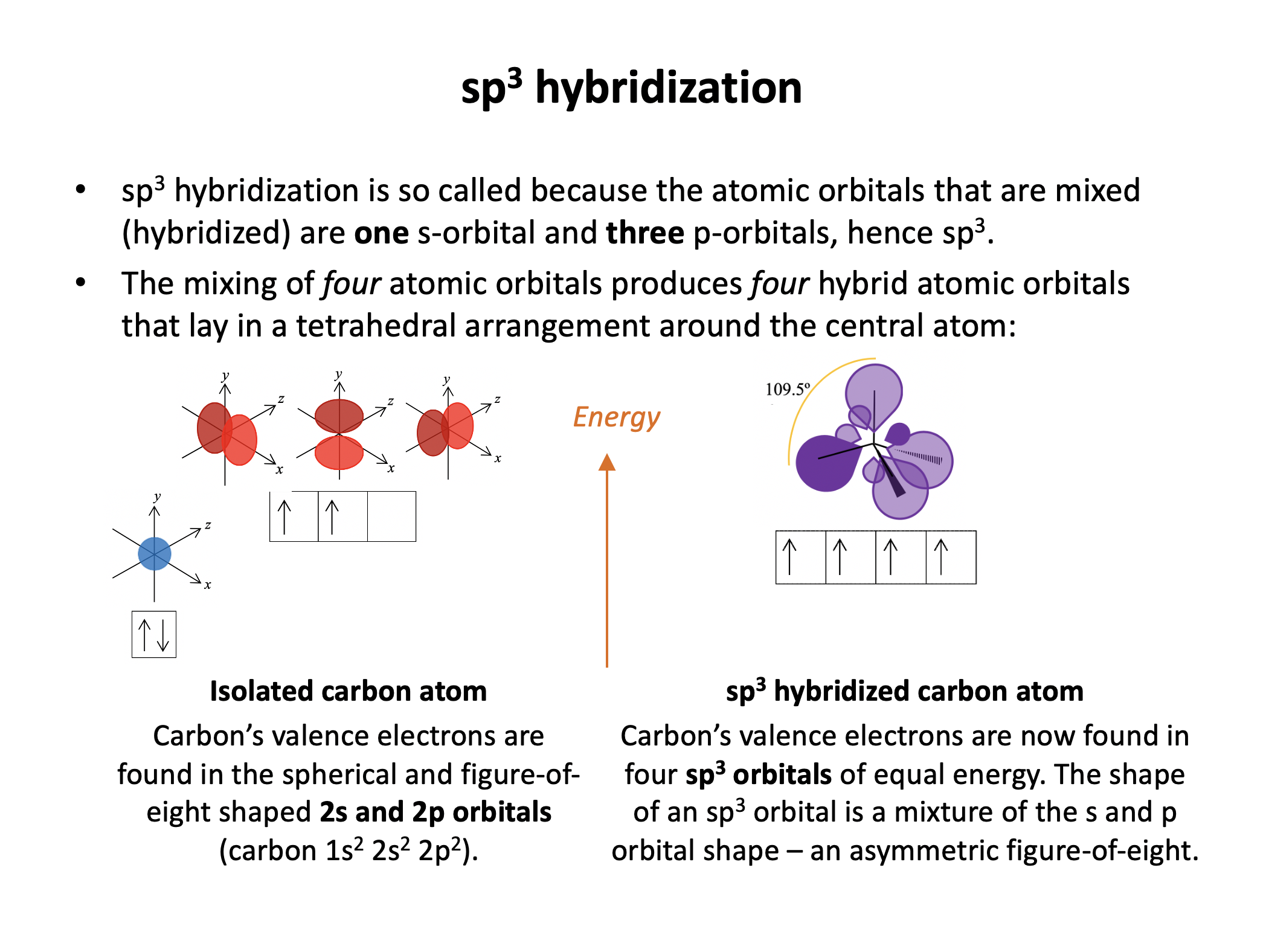
How many s orbitals and how many p orbitals combine to produce an sp3 hybrid orbital?
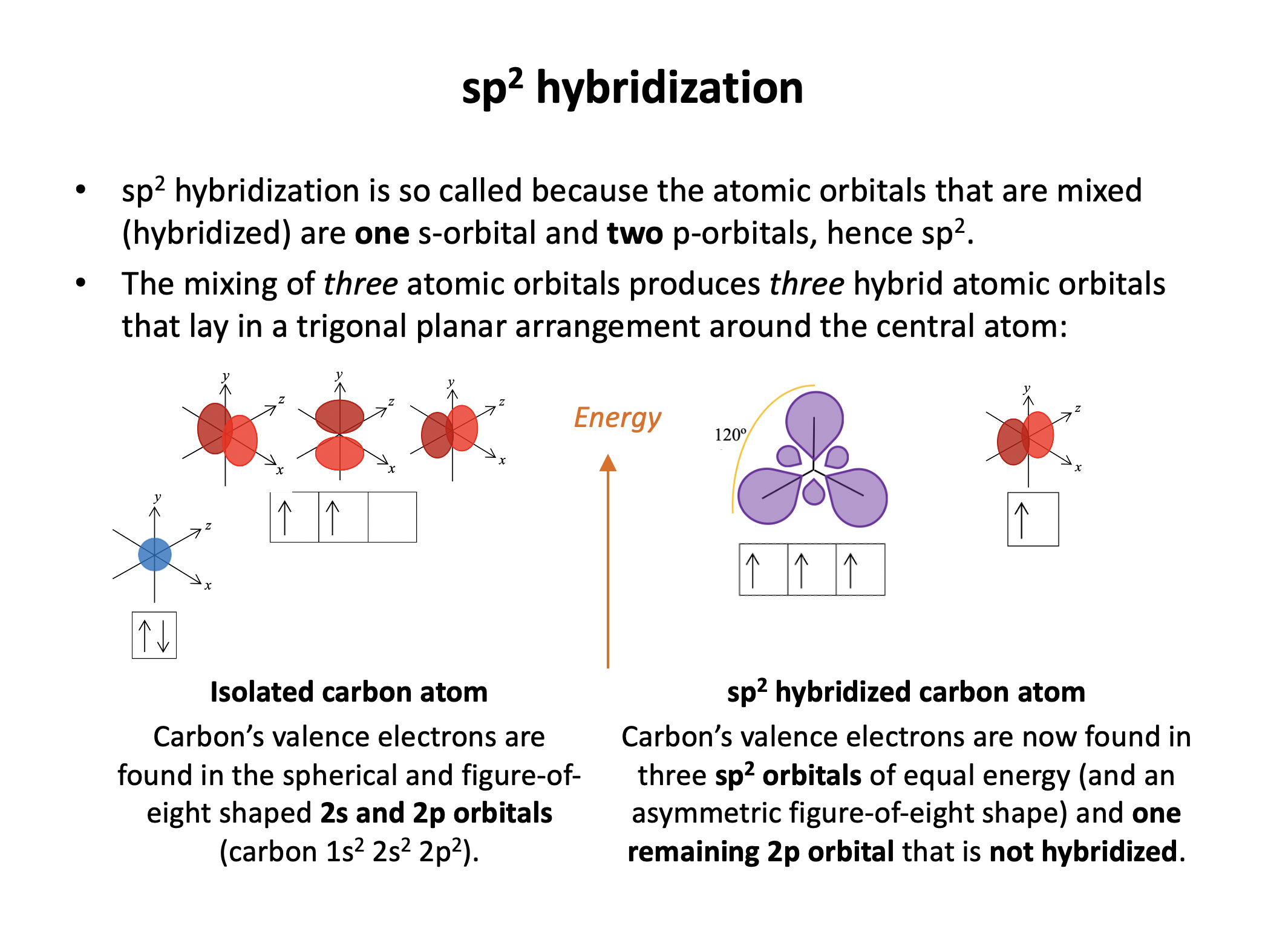
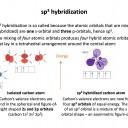
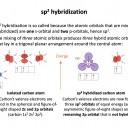
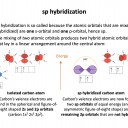
The hybrid orbital name gives this answer: sp3 has a 1 s orbital hybridising with 3 p orbitals. This, for example, occurs in a tetrahedrally bonded carbon atom.
Row A shows the electrons in the outermost atomic orbitals of an isolated C atom.
Row B shows the electrons in the hybrid orbitals when the four atomic orbitals have mixed to produce four hybrid (sp3) orbitals.
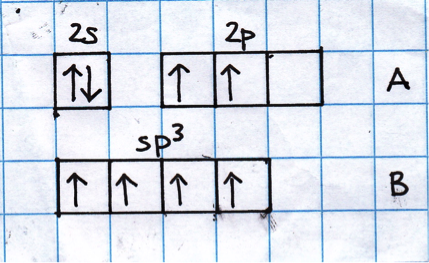
Look at the sketch below:

It represents the hybridisation of a carbon atom from an isolated state (row A), to a state in which the carbon is bonded tetrahedrally (row B).
Which of the following can be said to be true in going from A to B?
1: The total energy of the orbitals remains the same.
2: Each and every orbital has the same energy when in A and it does in B.
3: The orbitals in A and B have different shapes.
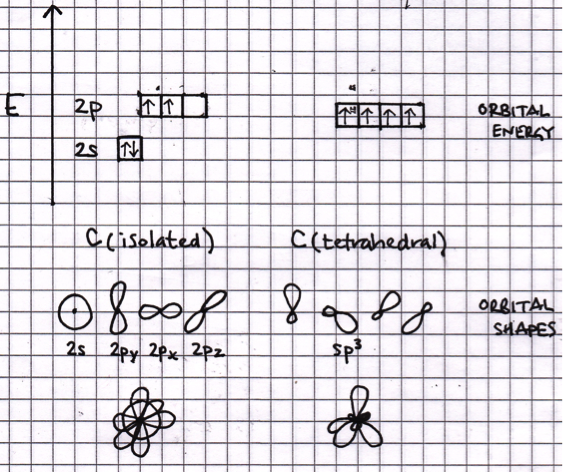
When the atomic orbitals of any atom hybridise (mix), the total energy of the orbitals remains the same. However, the energy of individual orbitals (that hybridise) will change, and the shapes of the orbitals (that hybridise) will change. See the sketch below:
Thus '1 and 3 only' is the correct answer.
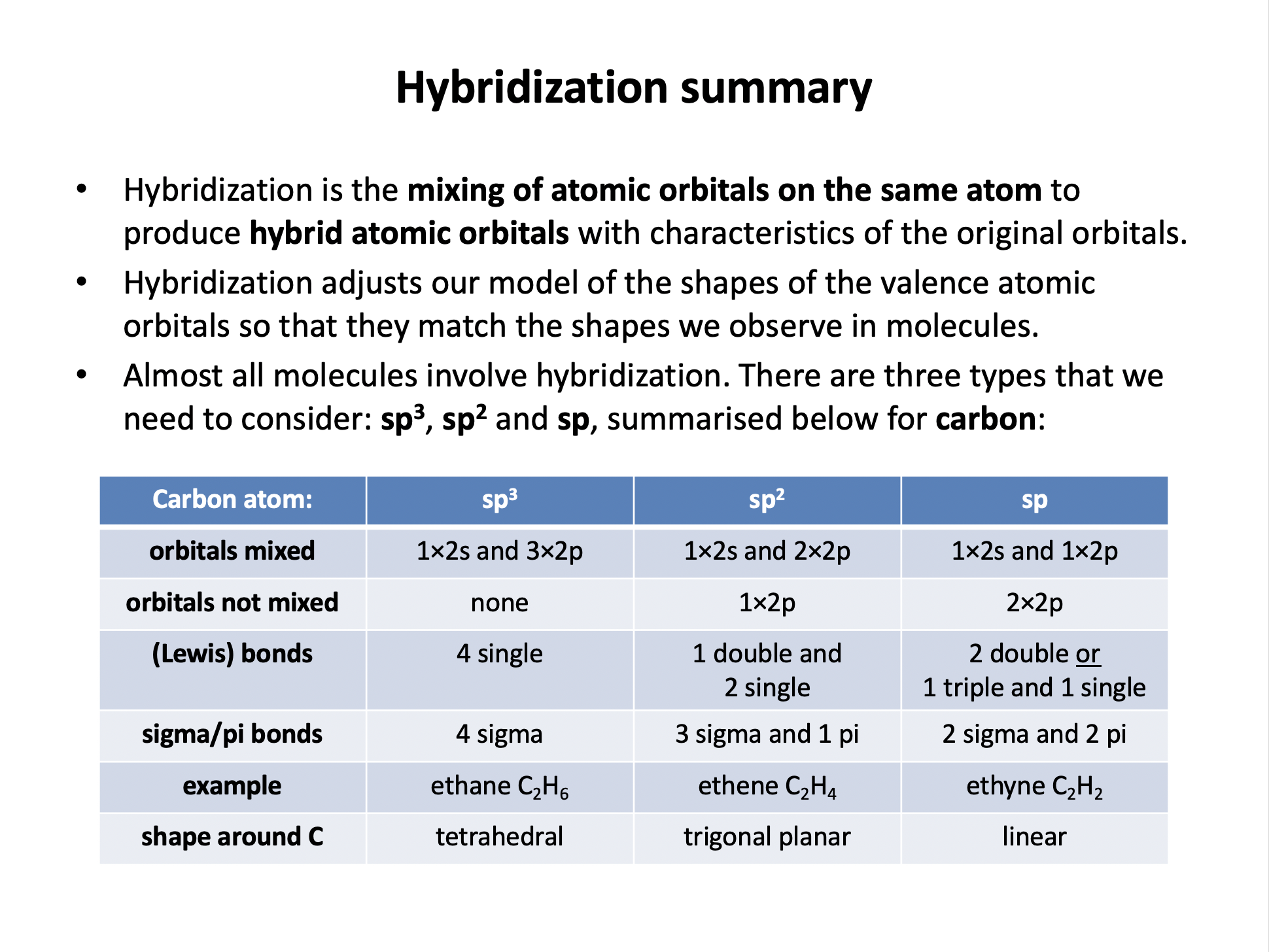
Consider propene CH3CHCH2. What is the hybridisation of each carbon atom in the order they are written?
The carbon-carbon bonds are C–C=C. The rule of thumb for remembering hydridisation in carbon:
All single bonds, carbon is sp3 hybridised.
One double bond (and two singles), carbon is sp2 hybridised.
One triple bond (and one single) or two double bonds, carbon is sp hybridised.
Thus the correct answer here is: sp3; sp2 ; sp2
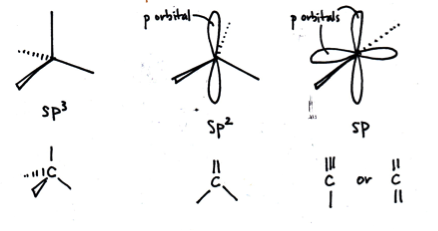
What are the shapes of the bonds around the carbon atoms in sp3, sp2 and sp hybridised carbons respectively?
The correct answer is 'tetrahedral ; trigonal planar ; linear'.
sp3 carbons have 4 single bonds arranged tetrahedrally; the four sp3 hydrid orbitals move as far apart as possible in 3D space (tetrahedral).
sp2 carbons have one double bond and 2 single bonds arranged in a trigonal planar shape; the three sp2 hydrid orbitals move as far apart as possible in 3D space (trigonal planar), and the non-hybridised p orbital sits above the plane forming a pi bond.
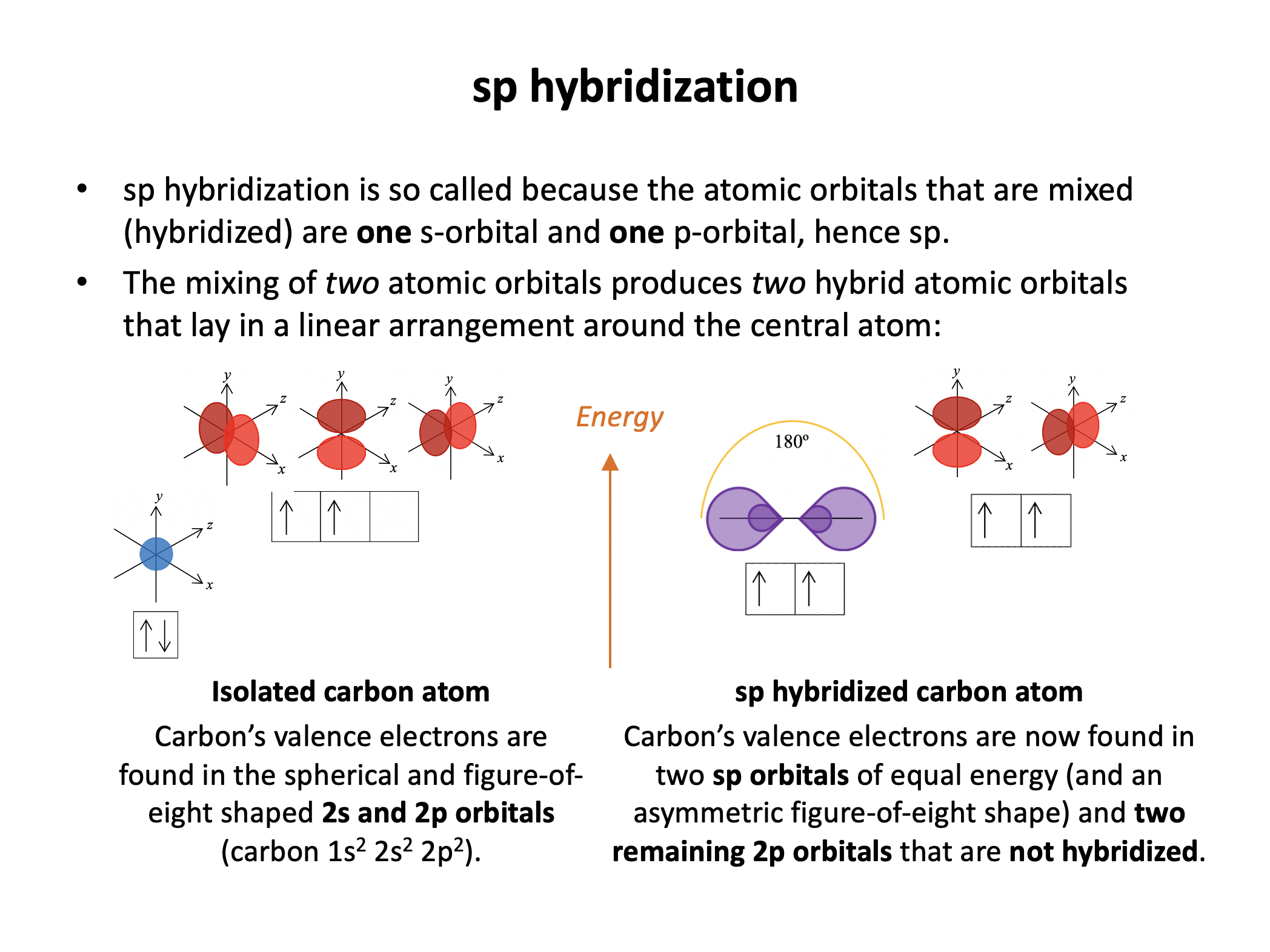
sp carbons have one triple bond and 2 single bonds (or two double bonds) arranged in a linear shape; the two sp hydrid orbitals move as far apart as possible in 3D space (linear), and the non-hybridised p orbitals sit perpendicular to the plane of the bonds, and each other, forming pi bonds.
In the diagram below hybrid orbitals are represented by lines (for clarity).
Which of the following describes the bonding in carbon dioxide CO2?
1: The carbon is sp hybridised.
2: The shape of the molecule is linear.
3: There are 2 sigma and 2 pi bonds in total in the molecule.
'1, 2 and 3' is the correct answer.
O=C=O; carbon dioxide has two double bonds. Each double bond consists of one sigma and one pi bond (so two of each in total).
The carbon atom is sp hybridised and has a linear shape around it (full explanantion below):
sp3 carbons have 4 single bonds arranged tetrahedrally; the four sp3 hydrid orbitals move as far apart as possible in 3D space (tetrahedral).
sp2 carbons have one double bond and 2 single bonds arranged in a trigonal planar shape; the three sp2 hydrid orbitals move as far apart as possible in 3D space (trigonal planar), and the non-hybridised p orbital sits above the plane forming a pi bond.
sp carbons have one triple bond and 2 single bonds (or two double bonds) arranged in a linear shape; the two sp hydrid orbitals move as far apart as possible in 3D space (linear), and the non-hybridised p orbitals sit perpendicular to the plane of the bonds, and each other, forming pi bonds.
In the diagram below hybrid orbitals are represented by lines (for clarity).

What is the hybridization of the nitrogen atom in aminomethane (below)?
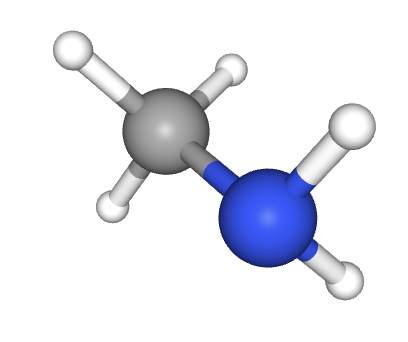
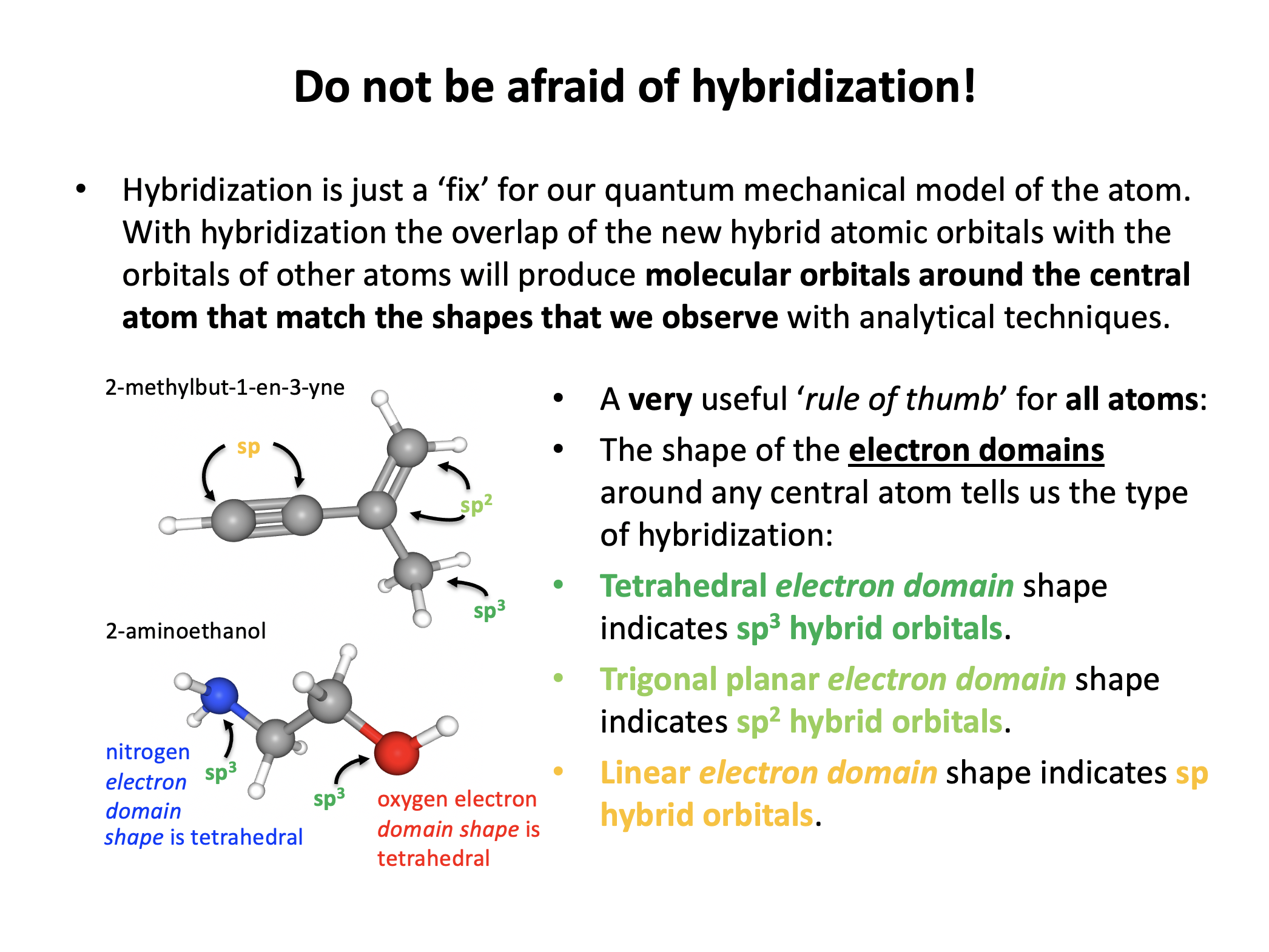
When identifying the hybridization of a given atom, it is best to use our 'rule-of-thumb'; that the geometry of the electron domains (see molecular shapes in the covalent structure section 4.3) tells us the hybridization:
Tetrahedral electron domains means sp3 hybridization.
Trigonal planer electron domains means sp2 hybridization.
Linear electron domains means sp hybridization.
The nitrogen atom here has electron domains that are tetrahedrally arranged (although the bonds are trigonal pyramidal around the central atom) so it must be sp3 hybridized.
sp3 hybridized is the correct answer.
What is the hybridization of the oxygen atom in propanone (below)?
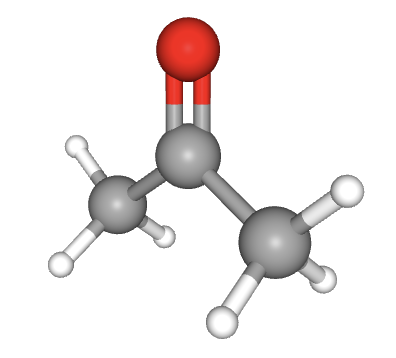
When identifying the hybridization of a given atom, it is best to use our 'rule-of-thumb'; that the geometry of the electron domains (see molecular shapes in the covalent structure section 4.3) tells us the hybridization:
Tetrahedral electron domains means sp3 hybridization.
Trigonal planer electron domains means sp2 hybridization.
Linear electron domains means sp hybridization.
The oxygen atom here has electron domains that are arranged in a trigonal planar manner so it must be sp2 hybridized.
sp2 hybridized is the correct answer.
Paper 1
Core (SL&HL): Bonding and Structure core (SL and HL) paper 1 questions
AHL (HL only): Bonding and Structure AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Bonding & Structure core (SL & HL) paper 2 questions
AHL (HL only): Bonding & Structure AHL (HL only) paper 2 questions
How much of Hybridization have you understood?






 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn