 This is a short section covering the structure of metals and metal alloys and corresponding properties. The content is relatively straightforward conceptually, but still needs to be learned.
This is a short section covering the structure of metals and metal alloys and corresponding properties. The content is relatively straightforward conceptually, but still needs to be learned.
Ensure you are confident using the terms below and learn the asterisked* definitions
a metallic bond*, electrostatic attraction, lattice (or giant) structure, non-directional bonds, an alloy, malleable, ductile.
What is a metallic bond?
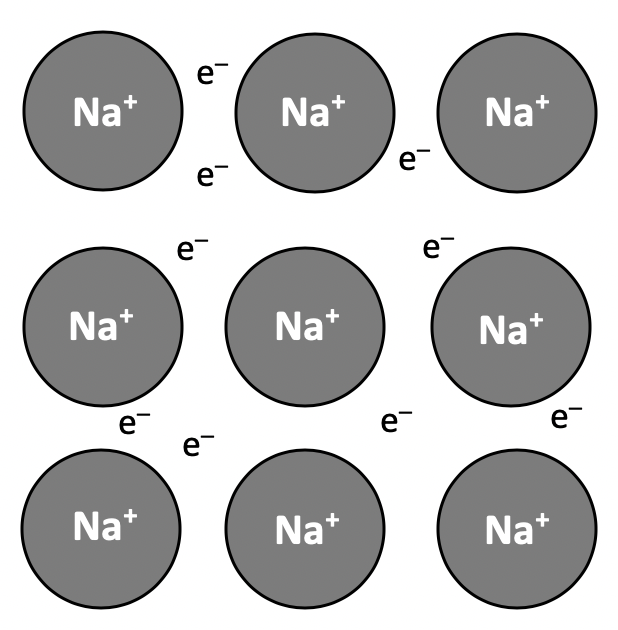
The correct answer is the electrostatic attraction between nuclei and delocalised electrons. Although this is often expressed as the electrostatic attraction between positive ions and (a sea of) delocalised electrons. Reference to the nuclei of the metal atoms or positive ions is equally valid (and both would be marked as correct in an examination).
The other explanations represent different types of bonding (covalent, ionic and intermolecular).
Which of the following metallic elements is likely to have the highest melting point?
Na; Mg; Al; K
Metals tend to have high melting points due to having giant (lattice) structures with many strong metallic bonds that need to be broken. The strength of the bond is related to the charge on the ion and the ionic radius.
Aluminium, with an ionic charge of 3+ and a small radius is likely to form the strongest bonds and have the highest melting point.
Which is the best description of why metals in the solid state can conduct electricity and why metals in the solid state are malleable (can bend and be shaped without breaking) respectively?
Metals conduct electricity in the solid state because the delocalised electrons (in the 'sea' of electrons surrounding the metal ions/atoms) are able to move and carry charge (the metal atoms/ions cannot move in the solid state).
Metals are malleable because the layers of atoms/ions are able to slide over one-another without breaking the electrostatic attraction between the nuclei and the delocalised electrons, as the metallic bonds attract in all directions (non-directional). Individual atoms/ions cannot move independently, but the layers can move when struck or bent.
The correct answer is therefore: 'The delocalised electrons are free to move: The layers of atoms/ions in the lattice can slide over one-another'.
Look at the table below:
Chemical | Melting/boiling point | Electrical conductivity (solid) | Electrical conductivity (molten) |
| W | high | high | high |
| X | high | nil | high |
| Y | high | nil | nil |
| Z | low | nil | nil |
Which of the four chemicals is likely to be a metal?
Metals are likely to have high melting and boiling points; they have giant (lattice) structures and it takes a lot of energy to break the network of strong metallic bonds. Metals conduct electricity in the solid state (and when molten) because the delocalised electrons can move and carry charge.
So W is the correct answer.
Which of the following properties of a particular metal can be altered by creating an alloy of the metal?
1: electrical conductivity
2: resistance to corrosion
3: melting point
A wide range of alloys have been developed that alter the properties of metals. Because of the non-directional nature of the attraction within the metallic lattice, is it relatively easy to introduce other metal atoms into the lattice to alter properties. 1,2 and 3 is the correct answer e.g.
Alloys of copper with some magnesium can increase the conductivity of copper.
Alloys of iron containing chromium and carbon form stainless steel that is resistant to corrosion.
Brass, an alloy of copper with some zinc, has a lower melting point than copper.
Paper 1
Core (SL&HL): Bonding and Structure core (SL and HL) paper 1 questions
AHL (HL only): Bonding and Structure AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Bonding & Structure core (SL & HL) paper 2 questions
AHL (HL only): Bonding & Structure AHL (HL only) paper 2 questions
How much of Metallic bonding have you understood?








 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn