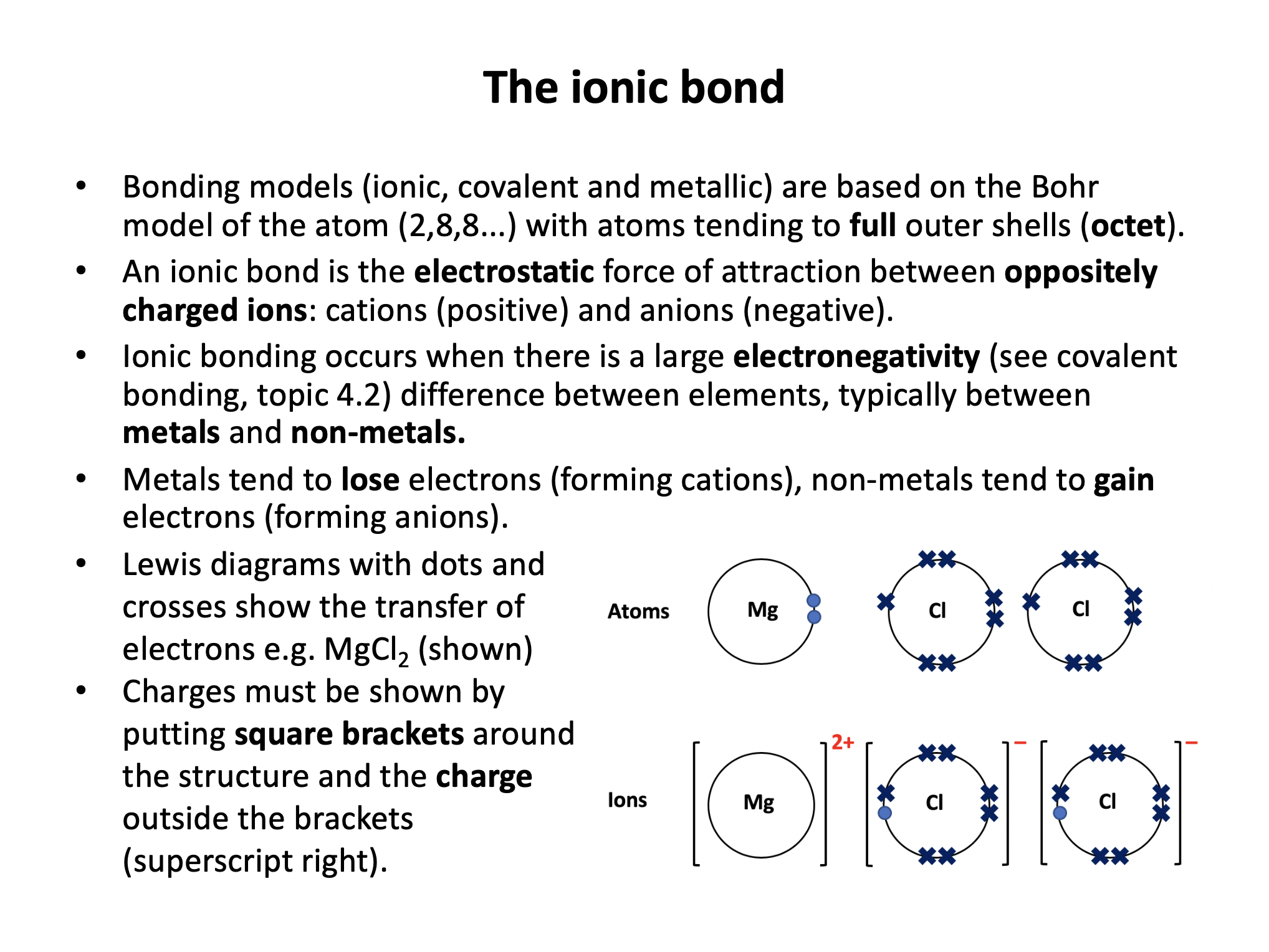
 Our model of ionic bonding is based on the Bohr model of the atom and represented with Lewis (dot-cross) diagrams. Ensure that you know the basics; how to work out the formulae of ionic compounds, including committing to memory the polyatomic ions. Don't forget to put your charges into your Lewis/dot-cross diagrams!
Our model of ionic bonding is based on the Bohr model of the atom and represented with Lewis (dot-cross) diagrams. Ensure that you know the basics; how to work out the formulae of ionic compounds, including committing to memory the polyatomic ions. Don't forget to put your charges into your Lewis/dot-cross diagrams!
Ensure you are confident using the terms below and learn the asterisked* definitions
an ionic bond*, an ion, an anion, a cation, electrostatic attraction, lattice (or giant) structure
What is an ionic bond?
The correct answer is the electrostatic attraction between oppositely charged ions. The other explanations represent different types of bonding (covalent, metallic and intermolecular).
When heated to high temperature in a lab, magnesium reacts with oxygen exothermically to produce magnesium oxide, a compound that is ionic.
What are the formulae for the magnesium and oxide ions in this ionic compound?
The charges on the ions in the main groups of the periodic table can be worked out by using the Bohr model of the atom:
| Group number - old (new system) | I (1) | II (2) | III (13) | V (15) | VI (16) | VII (17) |
| Electrons in outer (Bohr) shell | 1 | 2 | 3 | 5 | 6 | 7 |
| Charge on ion | 1+ | 2+ | 3+ | 3– | 2– | 1– |
| Example | Na+ | Mg2+ | Al3+ | N3– | O2– | Cl– |
Groups IV (14) and VIII (18) tend not to form ions. Note that the '1' is omitted when showing the charge on a 1+/– ion.
Magnesium is in group II and oxygen in group VI, so Mg2+ and O2– is the correct answer. (Indicating that each magnesium atom loses 2 electrons and each oxygen atom gains two electrons, obtaining a full valence shell of electrons.)
When heated to high temperature in a lab, aluminium reacts with oxygen exothermically, to produce aluminium oxide, a compound that is ionic.
What is the formula for aluminium oxide?
The charges on the ions in the main groups of the periodic table can be worked out by using the Bohr model of the atom:
| Group number - old (new system) | I (1) | II (2) | III (13) | V (15) | VI (16) | VII (17) |
| Electrons in outer (Bohr) shell | 1 | 2 | 3 | 5 | 6 | 7 |
| Charge on ion | 1+ | 2+ | 3+ | 3– | 2– | 1– |
| Example | Na+ | Mg2+ | Al3+ | N3– | O2– | Cl– |
Groups IV (14) and VIII (18) tend not to form ions. Note that the '1' is omitted when showing the charge on a 1+/– ion.
The aluminium ion is Al3+ and the oxygen ion is O2–, indicating that each aluminium atom loses 3 electrons and each oxygen atom gains two electrons obtaining a full valence shell of electrons. In order to balance the electrons lost and gained, therefore, two aluminium atoms will react with three oxygen atoms, hence the correct answer is Al2O3.
What is the correct formula for the nitrate ion?
The correct answer is NO3–. The other answers represent other ions (N3– is a nitride ion; NO2– the nitrite ion) or don't exist in a stable form (NO32–).
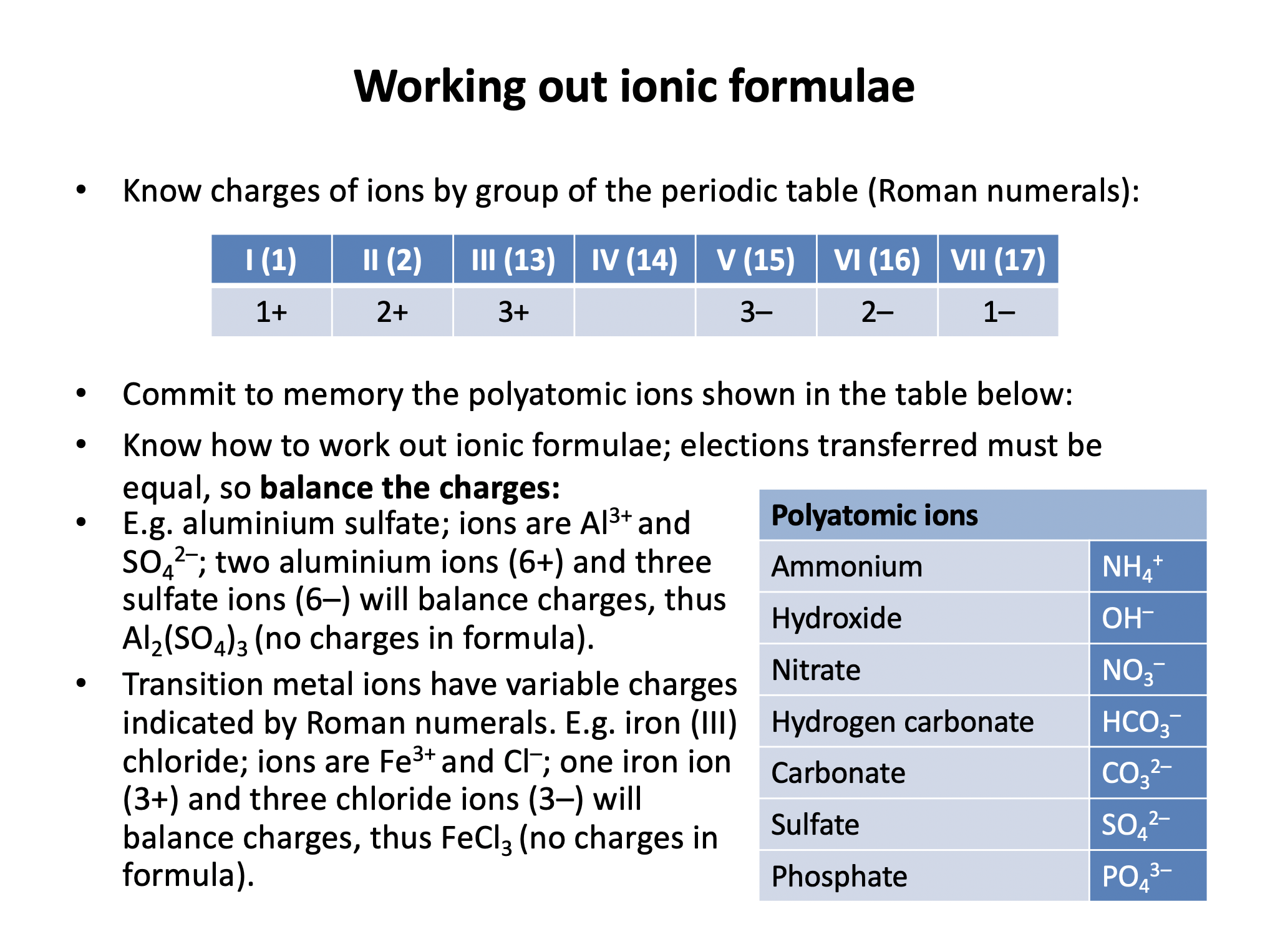
You do need to learn these polyatomic ions:
| ammonium | NH4+ |
| hydroxide | OH– |
| nitrate | NO3– |
| hydrogen carbonate | HCO3– |
| carbonate | CO32– |
| sulfate | SO42– |
| phosphate | PO43– |
What is the correct formula for magnesium hydroxide?
The correct answer is Mg(OH)2. The other answers represent compounds that don't exist in a stable form.
Remember that brackets are essential with polyatomic ions.
You do need to learn these polyatomic ions:
| ammonium | NH4+ |
| hydroxide | OH– |
| nitrate | NO3– |
| hydrogen carbonate | HCO3– |
| carbonate | CO32– |
| sulfate | SO42– |
| phosphate | PO43– |
Working out formulae with polyatomic ions is the same as with other ionic compounds (balancing electrons gained and lost) but remember not to confuse the number of atoms with the charge - e.g. the sulphate ion consists of 1 sulfur atom, 4 oxygen atoms, and has an overall charge of 2–.
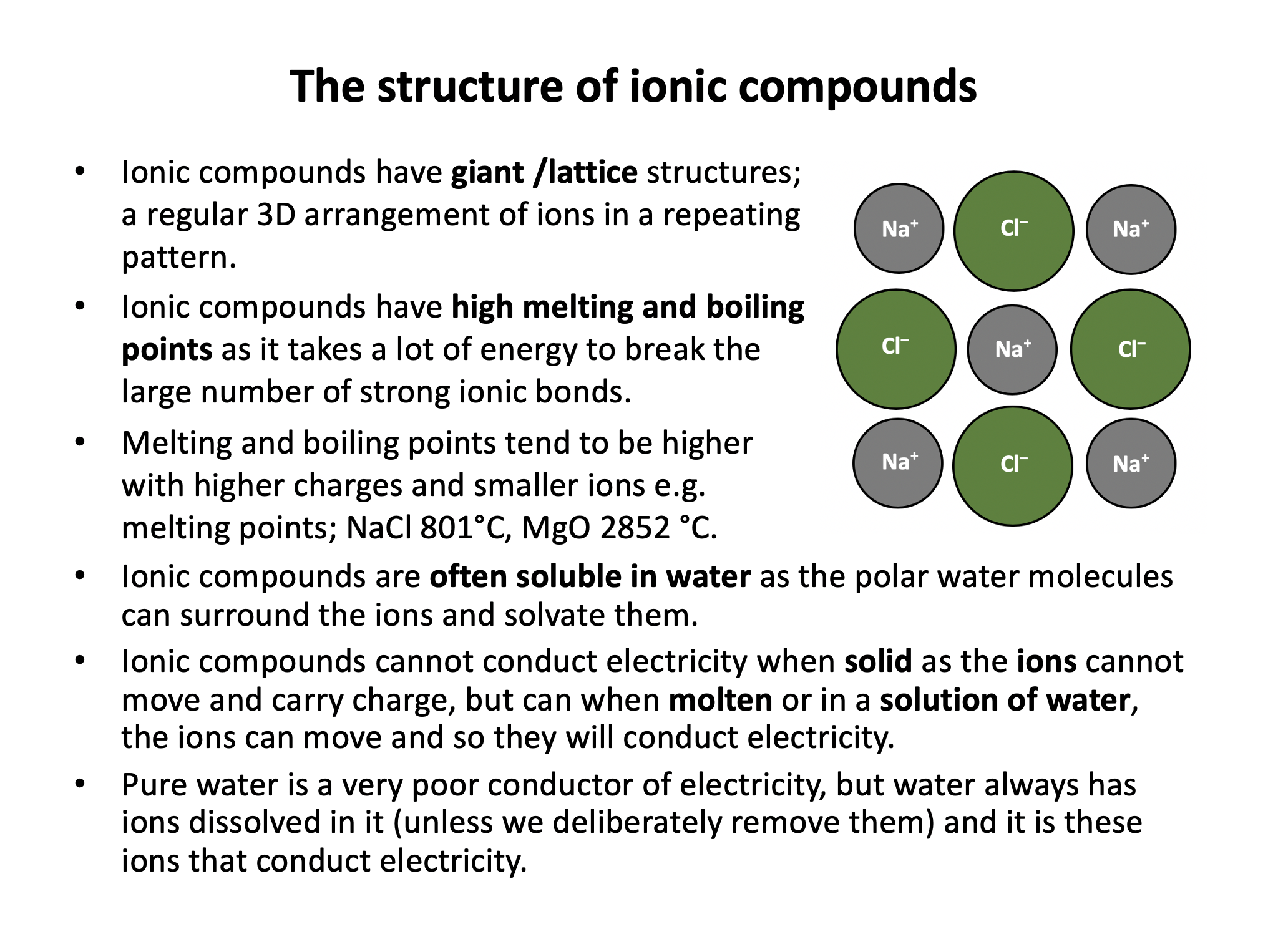
Which is the best description of the typical electrical conductivity of an ionic compound?
In a solid ionic compound the ions are fixed in position and cannot move, so solid ionic compounds do not conduct. In aqueous solution (water) or when molten the ions are free to move around, and can therefore carry charge and hence conduct electricity.
Do not conduct when solid, but do conduct in aqueous solution or when molten is therefore the correct answer.
Look at the table below:
Chemical | Melting/boiling point | Electrical conductivity (solid) | Electrical conductivity (molten) |
| W | high | high | high |
| X | high | nil | high |
| Y | high | nil | nil |
| Z | low | nil | nil |
Which of the four chemicals is likely to be an ionic compound?
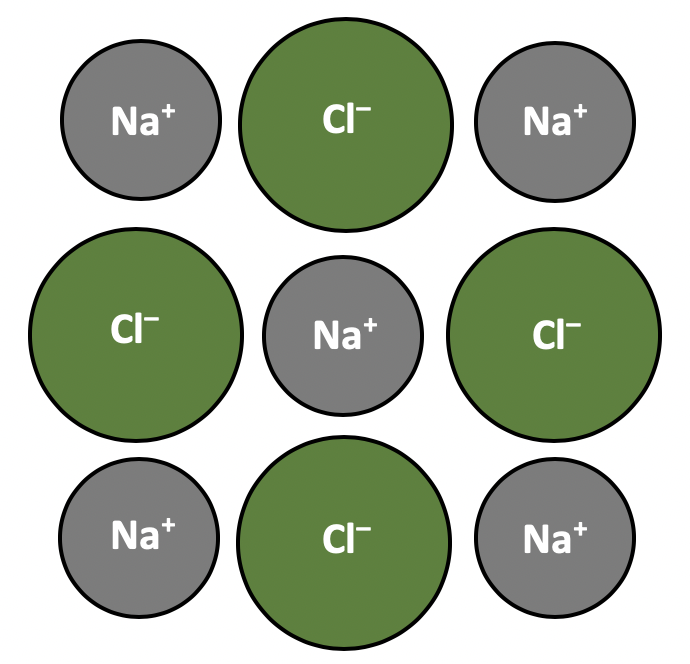
Ionic compounds are likely to have high melting and boiling points; they have giant (lattice) structures and it takes a lot of energy to break the network of strong ionic bonds. Ionic compounds do not conduct electricity in the solid state (ions cannot move) but do conduct when molten or in aqueous solution (ions can move and carry charge).
So X is the correct answer.
Paper 1
Core (SL&HL): Bonding and Structure core (SL and HL) paper 1 questions
AHL (HL only): Bonding and Structure AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Bonding & Structure core (SL & HL) paper 2 questions
AHL (HL only): Bonding & Structure AHL (HL only) paper 2 questions
How much of Ionic bonding and structure have you understood?








 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn