Solubility
Solubility related to functional groups
Once the naming of functional groups has been covered in sub-topic 10.1 Fundamentals of organic chemistry, teachers need to teach students how to relate properties such as volatility and solubility to the functional groups contained within compounds. This basically cross-links with sub-topic 4.4 Intermolecular forces.
For solubility there are essentially two concepts to consider. Firstly, how polar is the molecule in question? This depends both on shape and on the relative electronegativities of the atoms comprising the functional group. It also depends on the length of any hydrocarbon chains. Thus ethanol is very polar and therefore completely miscible with water whereas by the time the carbon chain has increased from two to five carbon atoms, as in pentan-1-ol, the compound is much less polar. So that pentan-1-ol, even though it still contains the –OH functional group, is hardly soluble in water at all.
The second concept addresses why polar molecules, such as ethanol, are completely miscible (i.e. very soluble) in water. It is not good enough just to say ‘like attracts like’. The reason is that the –OH functional group can hydrogen bond with water molecules. Molecules such as propanone can also form attractions with water molecules since the δ− on the oxygen atom of the carbonyl group is attracted to the δ+ hydrogen atoms of the water molecule. Non-polar organic compounds tend to be soluble in non-polar organic solvents such as hexane, ether or tetrachloromethane as they can form London (dispersion) forces of attraction between themselves and the non-polar solvent molecules.
As well as dealing with simple molecules containing up to six carbon atoms as the syllabus requires, these concepts can be re-enforced using interesting molecules in the data book. Examples include:
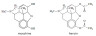
Example 1. Vitamin A and cholesterol (structures given in Sections 34 and 35).
Both contain the –OH functional group but both are insoluble in water due to the long hydrocarbon backbone.
Example 2. Vitamins
Vitamins can be divided into two separate groups - fat soluble and water-soluble. Ask students to look at the structures of vitamin A, vitamin D and vitamin C. All do in fact contain a polar –OH group. However students should soon see that vitamin A and vitamin D have very large non-polar hydrocarbon groups. These make the molecules virtually non-polar and both of these vitamins are fat soluble. Vitamin C on the other hand has many polar groups that can readily form intermolecular hydrogen bonding attractions to water molecules. This is why in the past sailors tended to suffer from scurvy – the result of vitamin C deficiency. The vitamin is excreted in urine and needs to be continually replaced. Without fresh fruit and vegetables sailors soon lacked sufficient vitamin C. A surgeon in the British Navy, James Lind, demonstrated this scientifically in the eighteenth century although it was not for another forty years that the navy adopted his findings. This is the origin of the slang name of ‘limeys’ for British sailors.
Example 3. Opiates
The structures of morphine and heroin have already been noted in the ‘salt formation’ page. Morphine is a diol and heroin is a diester. They are also both tertiary amines and can be made soluble in blood by being taken as their hydrochloride salts. Once in the body however they revert to their undissociated form. Because heroin is much less polar it is more soluble in non-polar lipids. This means it can penetrate the lipid-based blood/brain barrier more quickly than morphine which explains its greater potency.
Example 4. Sugars
Sugars are organic molecules and yet their solubility in water is well-known by students. The structures of glucose and fructose are given in Section 34 and it can be seen that they contain many polar –OH groups.
Example 5. Aspirin
Aspirin is not particularly soluble in water. It does contain polar groups but also a non-polar phenyl group. It can be made very soluble in water by making it into its soluble sodium salt by reacting the carboxyl functional group with sodium hydroxide to form the ionic sodium acetylsalicylate. This is detailed in the page on salt formation.

 IB Docs (2) Team
IB Docs (2) Team