Enantiomers
Background
Optical isomerism is not on the core part of the programme but is in the AHL under topic 20.3 Stereoisomerism. It also appears in the extension material of Option B under AHL topic B.10 Stereochemistry in biomolecules and in Option D under the AHL topic D.7 Taxol - a chiral auxiliary case study. When covering topic 20 with Higher Level students, teachers need to teach students how to use wedge-dash type representations involving tapered bonds to represent optical isomers. They are also advised to construct real or virtual 3-D models for of a wide range of stereoisomers, although no specific examples are given. Most teachers will probably use simple examples such as butan-2-ol, 2-bromobutane and/or lactic acid (2-hydroxypropanoic acid).
Personally, I think the use of real models such as molymod are crucial to the understanding of optical isomerism. By using models students can quickly grasp that enantiomers are only possible if there is a chiral carbon atom – i.e. one that has four different groups attached to it. However when I mark examination papers I am surprised how few students can correctly draw the 3-D structures of enantiomers even though they obviously understand the concept.
Ways to draw enantiomers correctly
There are two ways students can be helped to draw the two enantiomers correctly.
i. Draw one of the enantiomers showing the tetrahedral structure of the chiral carbon atom. For the second enantiomer keep two of the groups in exactly the same position and simply interchange the other two. (If more than two of the groups are interchanged it can be very easy to simply draw the same optical isomer twice).
ii. Probably the easiest way is to draw one of the enantiomers showing the tetrahedral structure of the chiral atom with the forward and backward groups on the right. Draw an imaginary (or real) mirror then draw the second enantiomer with the same two groups reflecting in the mirror on the left.
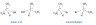
Once students have grasped the idea of chirality then the data book can be used as a source of many more interesting examples than just butan-2-ol, 2-bromobutane or 2-hydoxypropanoic acid. Even if they are not studying options B or D students are familiar with many of the substances contained within them and can relate to them. The following are some of the possibilities:
Examples from the data booklet
Example 1. 2-Amino acids
Simple condensation reactions between carboxylic acids and alcohols are part of the core content (10.2). Higher Level students studying Option A should know about the condensation reactions of carboxylic acids with amines to form condensation polymers and Standard Level students studying Option B cover the condensation reactions of amino acids to form proteins. Even though amino acids as such do not form part of the core or AHL programme they are important and it is not unreasonable to mention them in passing (particularly if the students are also studying Biology). Ask them to use Section 33 to identify the only amino acid that cannot show optical activity and draw the two enantiomers of alanine.
Example 2. Analgesics
Probably the one type of drug that all students will have taken is a pain-killer. Either aspirin, paracetamol (known as acetaminophen in the U.S.A.) or ibuprofen. Ask them to use the structures given in Section 37 to determine which one can show optical isomerism.

Only ibuprofen can exist as enantiomers
Example 3. Vitamins
The structures of three different vitamins are given in Section 35. Ask you students which can show optical isomerism. The prominent black wedge shown on the vitamin C structure will probably give the game away for vitamin C but how many will spot that vitamin D contains five different chiral carbon atoms?

Vitamin D has five chiral carbon atoms
Example 5. Straight chain sugars
Section 34 contains the structures of the open chain forms of both glucose and fructose. Glucose has four chiral centres whereas fructose only has three.
 Glucose (four chiral centres)
Glucose (four chiral centres)
 Fructose (three chiral centres)
Fructose (three chiral centres)
Example 6. Amphetamines
The 2014 data booklet contains the structures of other chiral compounds such as zanamivir and taxol (both in Section 37). Another class of drugs that they may not have taken but they will have heard of are the amphetamines. Amphetamines mimic the action of adrenaline – the flight or fight hormone. The structures of both amphetamine and adrenaline are no longer in the data booklet (they were in Table 20 of the 2009 booklet). They are given in my blog on Plant food or drug along with the structures of the illegal drugs ecstasy and mephedrone. You could show them the structures and ask the whether they can exist as enantiomers and to label the chiral carbon atom with an asterisk (*).


Both can exist as optical isomers but the chiral carbon atom is different in the two
molecules.

 IB Docs (2) Team
IB Docs (2) Team