2.1 The nuclear atom
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 2.1 The nuclear atom. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.
.png)

 Learning outcomes
Learning outcomes
After studying this topic you should be able to:
.png) Understand:
Understand:
- Atoms contain a dense positively charged nucleus which (except for hydrogen) is composed of protons and neutrons (nucleons).
- The space outside the nucleus is occupied by negatively charged electrons.
- The relative atomic mass of an element can be determined using a mass spectrometer.
Apply your knowledge to:
- Deduce the number of protons, neutrons and electrons in atoms and ions by using the nuclear symbol:

- Perform calculations involving non-integer relative atomic masses and abundance of isotopes from given data, including mass spectra.
Relationships & vocabulary
Nature of Science
This includes the importance of improvements in instrumentation to develop understanding of the structure of the atom such as the routes taken by alpha particles when fired at a thin sheet of gold which led to the Rutherford model of the atom replacing the 'plum pudding' model.
The work of Thomson and others showed that atoms can be broken down into smaller sub-atomic particles thus overthrowing the long held paradigm of atoms being indivisible.
International-mindedness
The enrichment of uranium by isotopic separation using the physical properties of the different isotopes of uranium operate in many different countries as part of nuclear energy and weaponry programmes.
For examples and more links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 2 & 12: The nuclear atom.
Vocabulary
| nucleon | isotope | radioisotope | mass number (A) | atomic number (Z) |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
Atomic theory is just that - a theory - but it is a very good one! Humans will never be able to see atoms as an atom is smaller than the wavelength of visible light. However scanning tunnelling microscopy does provide direct evidence to support the existence of atoms. This uses a very fine probe with a tungsten tip to scan a solid surface. It records the minute changes in current due to uneven surfaces when a potential difference is applied. This generates a contour map of the surface and the outlines of individual atoms can be detected.
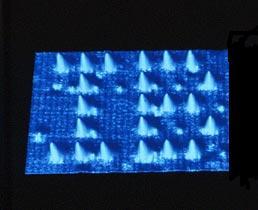
A scanning tunnelling microscope image of the IB made with xenon atoms on a nickel surface
(with apologies to IBM for losing their M).
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: The nuclear atom.
For short-answer questions see The nuclear atom questions.
More resources
1. You can download a useful simulation from PhET, University of Colorado at Boulder to show different Models of the hydrogen atom ![]() .
.
3. A useful video on how carbon dating works by Best of Science that explains it, puts it into context and also includes reference to other ways of dating artefacts.
4. One useful example to show how the physical properties of isotopes differ is the separation and enrichment of 235U from naturally occurring uranium (which is mainly 238U). This relies of the mass difference and the fact that UF6 is a gas so one of the methods of separation used is gaseous diffusion. Good information on uranium hexafluoride including how it can be separated into the different isotopes can be found in 'Molecule of the month' by Simon Cotton at the School of Chemistry, Bristol University.
5. A second example showing how the properties of isotopes differ using the fact that ice made from 'heavy water', D2O, sinks rather than floats as it is more dense than water.
6. Refer to my blog on isotopes in teeth to see how archaeologists can now determine human migratory patterns using isotopes of strontium.
7. Some further information on how scanning tunnelling microscopy works.

 IB Docs (2) Team
IB Docs (2) Team 


















