16.2 Activation energy
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 16.2 Activation energy. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.
 Learning outcomes
Learning outcomes
After studying this topic you should be able to:
 Understand
Understand
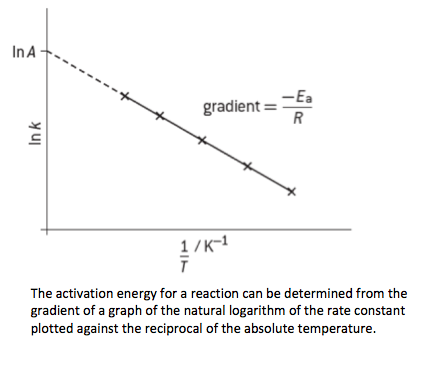
- The Arrhenius equation is based on the temperature dependence of the rate constant and can be used to determine the activation energy.
- A plot of 1/T against ln k produces a straight line with the gradient equal to – Ea / R and the intercept equal to ln A.
- A, the frequency factor (or pre-exponential factor), is related to the frequency of collisions with the correct orientations.
Apply your knowledge to:
- Use the Arrhenius equation in its normal form
k = A exp(–Ea/RT). - Analyse graphical representation of the Arrhenius equation in its logarithmic (linear) form
ln k = –Ea/RT + ln A. - Describe the relationships between temperature and rate constant, k; and between frequency factor, A, and the complexity of molecular collisions.
- Determine and evaluate the values of activation energy and frequency factors from data.
Relationships and vocabulary
Nature of science
Changing the temperature of a reaction has a much greater effect on the rate of reaction than can be explained by its effect on the rate of collisions. The Arrhenius equation, which proposes a quantitative model to explain the effect of temperature change on the rate of reaction, is a good example of how theories can be supported or falsified and replaced by new theories.
International-mindedness
For examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 6 & 16: Chemical kinetics.
Vocabulary
| frequency factor | Arrhenius equation | Arrhenius constant | ln (log to the base e) |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
The relationship between the value of k, the rate constant, and temperature is given by the Arrhenius equation:
k = A exp(–Ea/RT)
where k is the rate constant, A is another constant sometimes known as the Arrhenius constant but more usually as the frequency factor (or pre-exponential factor), Ea is the activation energy for the reaction, R is the gas constant and T is the temperature in Kelvin.
In theory you should know how to express this in logarithmic format but both the Arrhenius equation and its logarithmic form are provided in the Data booklet so you only really need to know how to use it. It is worth relating this to the Nature of Science (and to TOK) by stressing that this an example of an empirical relationship as it cannot be proven mathematically. Note that in the 'Something to think about' on the page for sub-topic 6.1 Collision theory & rates of reaction I have used the Arrhenius equation to show that the rule of thumb which says that raising the temperature by ten degrees doubles the rate of reaction depends very much upon the value of the activation energy.
 The equation is named after the Swedish chemist, Svante Arrhenius, who is perhaps more famous for being the first person to realise that electrolytes are dissociated into their positive and negative ions in aqueous solution and that the more dilute the solution the greater the degree of dissociation. There is a cautionary tale here – don’t let your teacher just dismiss your ideas out of hand. When Arrhenius submitted his work on the dissociation of electrolytes for his doctoral thesis at the University of Uppsala in 1884 the staff at the university thought his work was substandard and awarded him the lowest possible pass mark. Just 19 years later in 1903, after the brilliance of his revolutionary work on electrolytes had been fully recognized, he was awarded the Nobel Prize in Chemistry.
The equation is named after the Swedish chemist, Svante Arrhenius, who is perhaps more famous for being the first person to realise that electrolytes are dissociated into their positive and negative ions in aqueous solution and that the more dilute the solution the greater the degree of dissociation. There is a cautionary tale here – don’t let your teacher just dismiss your ideas out of hand. When Arrhenius submitted his work on the dissociation of electrolytes for his doctoral thesis at the University of Uppsala in 1884 the staff at the university thought his work was substandard and awarded him the lowest possible pass mark. Just 19 years later in 1903, after the brilliance of his revolutionary work on electrolytes had been fully recognized, he was awarded the Nobel Prize in Chemistry.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MCTest: Activation energy.
For short-answer questions see Activation energy questions.
More resources
1. A video by Richard Thornley on the Arrhenius equation and how to determine the activation energy for a reaction.
2. A simulation from the Wolfram Demonstration Project (you will need to download the free Wolfram CDF player to use it).
![]() Arrenhius equation for rate and velocity
Arrenhius equation for rate and velocity

 IB Docs (2) Team
IB Docs (2) Team 














