Factors affecting the rate of nucleophilic substitution reactions
This page explains how the nature of the halogen, whether the halogenoalkane is primary, secondary or tertiary and the choice of solvent can all affect the rate of substitution reactions of halogenoalkanes with nucleophiles such as the hydroxide ion. It reinforces chemistry given elsewhere on the site but in particular looks in detail at why protic polar solvents are preferred for SN1 reactions whereas aprotic polar solvents are preferred for SN2 reactions.
 The three factors affecting the rate
The three factors affecting the rate
Apart from the identity of the nucleophile itself, there are three main factors which affect the rate of nucleophilic substitution of halogenoalkanes.
1. The nature of the halogen
RX + NaOH → ROH + NaX (X = Cl, Br or I)
Iodoalkanes react faster than bromoalkanes which in turn react faster than chloroalkanes. This is due to the strength of the carbon to halogen bond. The C−I bond is the weakest and the C−Cl bond is the strongest so iodine is the better leaving group as less energy is required to break the carbon to halogen bond heterolytically. (Note that the C−F is so strong that fluoroalkanes do not undergo nucleophilic substitution reactions.)
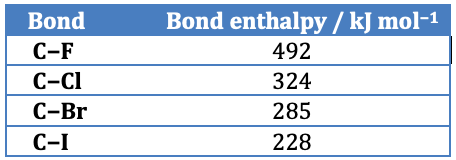
(Taken from Section 11 in the IB data booklet)
2. Whether the halogenoalkane is primary, secondary or tertiary
Nucleophilic substitution of tertiary halogenoalkanes proceeds by the SN1 mechanism so that the rate equation is rate = k[R−X] and does not involve the concentration of the nucleophile. The first (slow) step involves the breaking of the carbon to halogen bond to leave a carbonium ion (a positive carbon atom bonded to hydrogen atoms or other alkyl groups). The positive inductive effect of the surrounding alkyl groups increases the stability the intermediate carbonium ion making it more likely to form. Hence the order of reaction is:
tertiary halogenoalkane > secondary halogenoalkane > primary halogenoalkane
Note that primary halogenoalkanes proceed by the SN2 mechanism and secondary halogenoalkanes can proceed by both mechanisms. SN1 reactions are generally faster than SN2 reactions.
(See the slide gallery on 20.1(1) Nucleophilic substitution for fuller explanations of the first two factors and to refresh your understanding of the SN1 and SN2 mechanisms including the evidence for the SN2 mechanism from Walden inversion. You may also wish to refresh your understand of the correct use of 'Curly arrows'.)
3. The choice of solvent
This is the factor that students (and some teachers!) have the most problem with. Partly this is because the IB itself has been inconsistent with the way in which it is detailed in the chemistry guide.
In some ways it is not too easy to explain the choice of solvent at IB level as the only nucleophiles specifically mentioned in the syllabus are the hydroxide ion and water. Other nucleophiles such as cyanide ions, CN− and ammonia, NH3 are not referred to in the guide. Similarly no specific examples of protic and aprotic solvents are given. What students need to know is that both SN1 and SN2 reactions are best carried out in polar solvents but protic solvents should be used for SN1 reactions whereas aprotic solvents should be used for SN2 reactions. According to the guide you do not need to be able to explain this as under the ‘Application and skills” part of 20.1 you are only required to be able to outline the difference between protic and aprotic solvents rather than explain why different solvents affect the rate of nucleophilic substitution. However it may well help you in your understanding if an explanation is given.
The reason why polar solvents are required for both SN1 and SN2 reactions is that they help to stabilises the carbocation intermediate in SN1 reactions and the transition state in SN2 reactions as both involve ions. They also help to dissolve the nucleophile as nucleophiles either have a negative charge (e.g. OH−) or are polar themselves (e.g. H2O or NH3). However the more polar the solvent the more it solvates the nucleophile which paradoxically makes the nucleophile less nucleophilic. This is not a problem in SN1 reactions as the first step does not involve the nucleophile. The nucleophile is attracted to the carbonium ion intermediate once it has been formed in the first slower step. In SN2 reactions though the nucleophile is involved in the first (slow) step where it is attracted to the central carbon atom which only has a small positive charge.
The difference between protic and aprotic is basically the ability of the solvent to form hydrogen bonds. Protic solvents such as water and ethanol contain O−H bonds and the proton develops a significant positive charge due to the much greater electronegativity of the oxygen atom. The protic hydrogen can strongly interact with anions, whereas the non-bonding pair of electrons on the oxygen atom can stabilize cations. Polar aprotic solvents, such as propanone, CH3COCH3, are still polar enough to dissolve charged species (such as halide and hydroxide ions) but do not form hydrogen bonds as they possess no δ+ protons. In SN1 reactions polar protic solvents can help to stabilise the leaving group so that the nucleophile is able to interact with the intermediate carbocation. Aprotic solvents can still help to dissolve the nucleophile but do not solvate the nucleophile as much as protic solvents so in SN2 reactions the nucleophile can be attracted to the central δ+ carbon atom to form the transition state with five groups surrounding the central carbon atom.

 IB Docs (2) Team
IB Docs (2) Team