14.2 Hybridization
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 14.2 Hybridization. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.
.png)
 Learning outcomes
Learning outcomes
.png) After studying this topic you should be able to:
After studying this topic you should be able to:
Understand:
- Hybrid orbitals result from mixing different types of atomic orbitals on the same atom.
Apply your knowledge to:
- Explain the formation of sp, sp2 and sp3 hybrid orbitals in ethyne, ethene and methane.
- Identify and explain the relationships between Lewis (electron dot) structures, electron domains, molecular geometries and the different types of hybridization.
Relationships & vocabulary
Nature of science
Theories are often uncertain. For example, hybridization is part of valence bond theory and can help to explain molecular geometries and some chemical behaviour but is limited. Another example is quantum mechanics which involves several theories. These can explain the same phenomena but depend upon specific requirements.
International-mindedness
For examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 4 & 14: Chemical bonding & structure.
Vocabulary
| hybridzation | sp | sp2 | sp3 |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
The concept of hybridization is a useful model to explain the shape, bond angles and Lewis structure of molecules. It was developed by Linus Pauling in 1932, the same year that he introduced the idea of electronegativity. I first came across it at university and it has a somewhat chequered history in pre-university syllabuses. During the 1970s and 1980s it appeared on British A level syllabuses but it has subsequently disappeared from most pre-university syllabuses with the IB being somewhat of an exception.
Essentially hybridization describes the combination of atomic orbitals to form new hybrid atomic orbitals with the same mean energy. In the IB this is limited to sp, sp2 and sp3 but at a university level it is also involves the hybridization of d orbitals. It can be used to describe shapes in coordination complexes of transition metal compounds such as [Mn(H2O)6]2+ where the hexagonal-shaped complex ion can be explained by invoking d2sp3 hybridization on the metal ion.
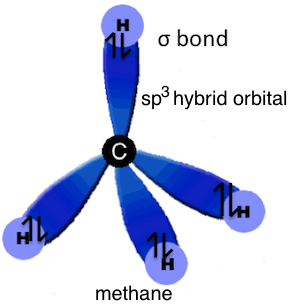
sp3 hybridization used to explain the symmetrical tetrahedral shape of methane where a single electron in each of the sp3 hybrid orbitals on the carbon atom forms a sigma bond with the single s electron in each of the four hydrogen atoms.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Hybridization.
For short-answer questions see Hybridization questions.
More resources
1. One of the better videos on hybridization (there are many bad ones on YouTube!). This explains sp (BeCl2), sp2 (benzene) and sp3 (methane) hybridization correctly in terms of energy as well as shapes.
2. A video that shows rotating models of the different types of hybridization between s and p orbitals.

 IB Docs (2) Team
IB Docs (2) Team 












