A.3 Catalysts
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Option A - sub topic A.3. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.
.png)

 Learning outcomes
Learning outcomes
After studying this topic students you be able to:
.png) Understand:
Understand:
- In heterogeneous catalysis the reactants adsorb at active sites and the products desorb.
- In homogeneous catalysis the catalysts chemically combine with the reactants to form temporary activated complexes or reaction intermediates.
- The catalytic properties of transition metals depend upon the adsorption/absorption properties of the metals and their variable oxidation states.
- Zeolites can act as selective catalysts due to their cage structure.
- Catalytic nanoparticles have a large surface area per unit mass.
- Explain the factors involved in choosing a suitable catalyst for a particular process.
- Describe how metals work as heterogeneous catalysts.
- Describe the benefits of nanocatalysts in industry.
Relationships & vocabulary
Nature of science
Catalysts were used to increase the rates of reactions long before it was understood how they work. Developments in the mechanism of catalysis have led to the use of models that are constantly being tested and improved.
International-mindedness
The theft of catalytic converters is on the increase globally due to the value of the precious metals, rhodium, palladium and platinum, which are commonly used as the catalysts in these converters.
Vocabulary
| adsorb / adsorption | absorb / absorption | desorb |
| heterogeneous | homogeneous | zeolite |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
It is fairly easy to see how a heterogeneous catalyst works. Two reacting gases, e.g. hydrogen and ethene, adsorb onto the surface of a transition metal such as nickel or palladium. This brings the two reactants close together and with the right orientation producing an alternative pathway with a lower activation energy than the uncatalysed reaction. Once the product has formed, it is then desorbed. It is less easy to find good examples of how homogeneous catalysts work. One that is often given (and I use in my Study Guide) is the reaction between peroxodisulfate(VI) ions and iodide ions, which is catalysed by iron(II) ions. It is thought that the Fe2+(aq) ions are oxidised to Fe3+(aq) ions and then back to Fe2+(aq) ions.
S2O82−(aq) + 2Fe2+(aq) → 2SO42−(aq) + 2Fe3+(aq)
2I−(aq) + 2Fe3+(aq) → 2Fe2+(aq) + I2(aq)
Although this shows that the oxidation state of the iron changes, it does not show any intermediate compound formation. One really good example to use is slightly esoteric as it involves a transition metal complex with a ligand that you will be unfamiliar with. This ligand is triphenylphosphine, P(C6H5)3 – shortened to PPh3. However the chemistry is easy to follow and students should recognise the phenyl functional group. The catalyst, chlorotris(triphenylphosphine)rhodium(I), is known as ‘Wilkinson’s compound’ named after Professor Sir Geoffrey Wilkinson (1921-1996) who discovered it and who went on to win the Nobel Prize in 1973 for his work on transition metal complexes. It has the formula RhCl(PPh3)3 and is a good homogeneous hydrogenation catalyst for alkenes.
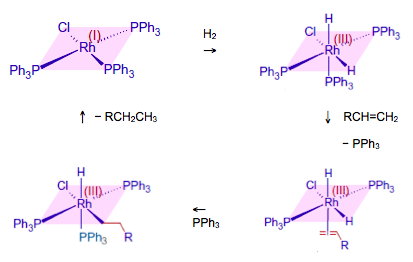
Proposed mechanism for hydrogenation of an alkene catalysed by RhCl(PPh3)3
Chlorotris(triphenylphosphine)rhodium(I) is a square planar complex. The triphenylphosphine is analogous to ammonia so behaves as a neutral ligand. You should be able to confirm that the oxidation state of rhodium in the compound is +1, as shown by its name. In solution hydrogen adds to the complex to form an octahedral complex. This is known as oxidative addition as the oxidation state of the rhodium has increased to +3. One of the triphenylphosphine ligands is now replaced by an alkene, which bonds via the pi electrons in the double bond. This brings the alkene close to the hydrogen atoms and it is thought that the rate-determining step is the addition of one of the hydrogen atoms to the alkene followed by the insertion of a triphenylphosphine molecule into the vacant site left by the hydrogen atom. The alkyl radical then reacts with the remaining hydrogen atom and leaves in a process known as reductive elimination to regenerate the original rhodium(I) compound.
Intermediates such as those shown above are obviously highly reactive so it is impossible to prove any suggested mechanism by isolating all the intermediates, although chemists have come up with several ingenious ways in which their presence can either be shown or inferred.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) MC test: Catalysts.
For short-answer questions see Catalysts questions together with the worked answers on a separate page Catalysts answers.
More resources
1. A down to Earth description of what a catalytic converter is and how it works. It is worth putting up with the miaow to start with!
2. A short but informative video on what zeolites are and what they can be used for by Jeff Rimer of the University of Houston Cullen College of Engineering.
3. A good overview of the use of nanoparticles including their use as catalysts in fuel cells from Bytesize Science. To show how fast the technology is changing it shows an "old" inefficient fuel cell based on platinum (which is all of eleven years old!) which produces 100 W and compare it to a modern fuel cell using nanoparticle sized catalysts that produces 1 kW.
![]() Developments in nanotechnology
Developments in nanotechnology

 IB Docs (2) Team
IB Docs (2) Team 
















