B.4 Carbohydrates
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Option B - sub topic B.4. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.

 Learning outcomes
Learning outcomes
After studying this topic students you be able to:
 Understand:
Understand:
- The general formula of carbohydrates is Cx(H2O)y.
- The cyclic structures of monosaccharides can be represented by Haworth projections.
- Monosaccharides contain several –OH groups and either an aldehyde group (aldose) or a ketone group (ketose).
- In solution, the straight chain forms of sugars cyclize to form ring structures which contain an ether linkage.
- Disaccharides and polysaccharides are formed when monosaccharides condense forming glycosidic bonds.
- Carbohydrates are used as energy sources and energy reserves.
- Deduce the structural formulas of disaccharides and polysaccharides from given monosaccharides.
- Relate the properties and functions of monosaccharides and polysaccharides to their chemical structures.
Relationships & vocabulary
Nature of science
Understanding the stereochemistry of carbohydrates is the key to understanding their structural roles in cells. By making carbon and hydrogen implicit, models using Haworth projections help to focus on the nature and position of attached groups.
International-mindedness
Sugar is a major international commodity that is produced in about 130 different countries. About three-quarters of the production comes from sugar cane in tropical and subtropical regions with the remainder coming from sugar beet that is cultivated in temperate climates.
The World Health Organization projects that deaths from diabetes (a chronic disease that occurs when the body cannot effectively regulate blood sugar, due to a failure in the production or functioning of insulin) will double between 2005 and 2030.
Lactose intolerance is due to a failure to produce sufficient levels of lactase, the enzyme that hydrolyses lactose into glucose and galactose. Globally, lactose intolerance (a condition in which the individual is not able to digest lactose, the sugar found in milk and dairy products) is the norm and provides an example of a Western perspective invading science.
Vocabulary
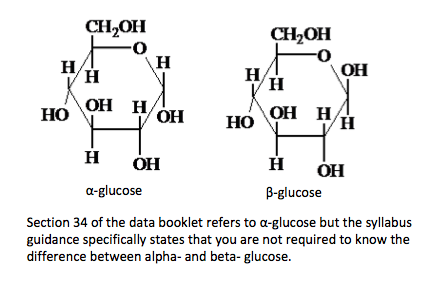
| monosaccharide | disaccharide | polysaccharide | α-glucose and β-glucose |
| starch | amylose | amylopectin | cellulose |
| dietary fibre | glycosidic bond | Fischer & Haworth projections |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
Stereochemistry of glucose
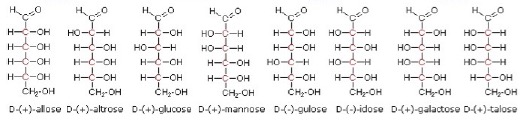
Although glucose is a simple sugar and a well-known compound, its structure and how it can be represented does pose some problems. It contains four chiral centres. This means that theoretically 24 (i.e. 16) stereoisomers are possible, arranged in eight diastereoisomeric pairs. This was first recognised by Emil Fischer, a German chemist, who won the Nobel Prize for chemistry in 1902. Fischer arbitrarily labelled any glucose structure where the –OH bonded to the last chiral carbon atom next to the aldose group (furthest from the aldehyde group) on the right as the D- form. This is for convenience and does not relate to whether the stereoisomer is the d- or l- form in terms of the rotation of plane polarized light. The eight possible stereoisomers of the D form, drawn in what are known as Fischer structures are thus:
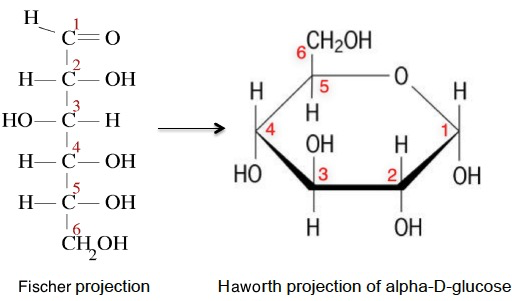
In solution the –OH group on the fifth carbon atom forms an ether linkage with the first carbon atom and the carbonyl group is converted into an alcohol. If the –OH group formed on the first carbon atom is on the opposite side of the ring to the sixth carbon atom then it is known as the alpha- form.
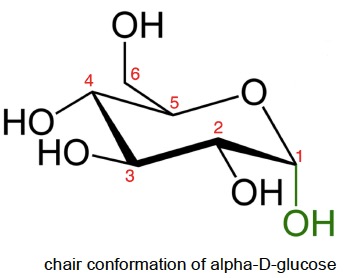
The syllabus uses the Haworth projection, which is a convenient way to show the stereochemistry. The ring drawn in the chair conformation shows both the conformation and stereochemistry but is not required by the IB.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Carbohydrates.
For short-answer questions see Carbohydrate questions together with the worked answers on a separate page Carbohydrate answers.
More resources
1. A simple video (possibly made by students) but it provides a good illustration of the structures of glucose and condensation reactions to form starch, glycogen and cellulose.
2. A lecture on the ring structures of sugars including the Haworth projection.
3. A link to a website from Elmhurst Collge that helps to distinguish clearly between the amylose and amylopectin forms of starch. It also contains links to other web pages on carbohydrates.

 IB Docs (2) Team
IB Docs (2) Team 












