8.3 pH scale
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 8.3 pH scale. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.


 Learning outcomes
Learning outcomes
 After studying this topic you should be able to:
After studying this topic you should be able to:
Understand:
- pH = ﹣log10[H+(aq)] and [H+(aq)] = 10﹣pH .
- A change of one unit of pH represents a tenfold change in the hydrogen ion concentration, [H+(aq)].
- Acidic, neutral and alkaline soutions can be distinguished using the pH scale.
- The ionic product constant for water,
Kw = [H+(aq)] x [OH﹣(aq)] = 1.00 x 10﹣14 at 298 K.
Apply your knowledge to:
- Solve problems involving pH, [H+] and [OH﹣].
- Gain familiarity with the use of a pH meter and universal indicator.
Relationships & vocabulary
Nature of science
The syllabus implies that the pH scale is an example of Occam's razor as it is an attempt to scale relative acidity over a wide range of H+ concentration into a relatively simple number. This seems completely wrong as Occam's razor is concerned with the simplest theory or explanation whereas the pH scale is neither of these. It is simply a logarithmic scale of the hydrogen ion concentration in aqueous solution.
International-mindedness
For examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 8 & 18: Acids & bases.
Vocabulary
| pH | universal indicator | hydrogen ion concentration, [H+(aq)] | ionic product constant of water, Kw |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
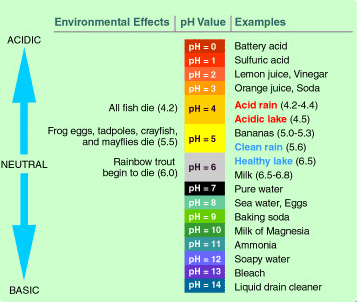
The pH scale goes from 0 to 14
A question to consider: Does 10.0 mol dm-3 hydrochloric acid solution (see below) have a pH of minus 1?
 In this sub-topic pH is defined as being equal to minus the logarithm to the base ten of the hydrogen ion concentration, i.e.
In this sub-topic pH is defined as being equal to minus the logarithm to the base ten of the hydrogen ion concentration, i.e.
pH = – log10[H+(aq)]
For hydrogen ion concentrations between 1.00 mol dm-3 (pH = 0) and 1.00 x 10-14 mol dm-3 (pH = 14) there is no contradiction between this definition and the fact that the pH scale goes from 0 to 14. However what happens when we have a hydrogen ion concentration of 2.0 mol dm-3 ? This is actually the case with normal bench 'dilute' hydrochloric acid solution. Now the pH = – log10 2.0 which is equal to – 0.30. Similarly the pH of 10.0 mol dm-3 hydrochloric acid solution will be ﹣1 according to the definition. One of the statements must be wrong. Either the pH can have a value below zero - and above 14 (in the case of 2.0 mol dm-3 NaOH(aq) for example) - or the lowest value it can have is zero.
There would appear to be two reasons why pH does not have a negative value. Hydrochloric acid is a strong acid, i.e. it is completely dissociated into its ions in aqueous solution. Perhaps this assumption is wrong for more concentrated solutions. If we have 2.0 mol dm-3 hydrochloric acid solution it does not follow that it is completely dissociated since Ostwald's dilution law which is for weak electrolytes (such as ethanoic acid) and states that the more dilute the solution the greater the degree of dissociation may also apply to more concentrated solutions of strong acids. Certainly if water is added to quite concentrated sulfuric acid (don't do this in practice! - the short (5 seconds!) video below shows the safer practice of the acid being added to water) a considerable amount of heat is given out as the ions become hydrated so clearly some reaction is taking place when solutions of concentrated strong acids are diluted.
The second reason is that although the mathematical statement pH = – log10[H+(aq)] is correct, the word definition that pH equals minus the logarithm to the base ten of the hydrogen ion concentration is not correct because the square brackets do not actually represent concentration. The square brackets around the hydrogen ion, [H+(aq)], actually refer to the activity not the concentration. For dilute solutions the activity is virtually the same as concentration but for more concentrated solutions it is considerably less and hence the value of – log10[H+(aq)] does not drop below zero. Who said Chemistry is easy?
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: The pH scale.
For short-answer questions see pH scale questions.
More resources
1. A basic outline of the pH scale with examples of substances with different pH values over the whole pH range
2. This video is also fairly simple but does explain how the scale arises using the definition of pH and the hydrogen ion concentration.

 IB Docs (2) Team
IB Docs (2) Team 
















