NoS question - Example 1
Background information
Perhaps one of the easiest parts of Nature of Science to assess is the validity of a hypothesis. The use of hypotheses is covered in the Nature of Science part of the chemistry guide which deals with the Understanding of Science. Hypotheses are explanatory statements about the world. They may be true or false but should be based on solid reasoning rather than pure guesswork. A hypothesis may suggest a causal relationship or a correlation between factors. Support of or opposition to a hypothesis can be tested by experimental observations of the natural world.
The validity or otherwise of a hypothesis has been assessed several times in the past. Question 2 on TZ1 2009 SL Paper 2 (it is Question 3 on the HL Paper) is a good example. Even though I wrote this question I cannot repeat it here as it is copyright IB but your teacher should easily be able to get hold of it. What I have done for Example 1 is to write another question which is essentially about Topics 5. Energetics but contains a considerable amount of Nature of Science and also contains some of the new command terms that first appeared on the current programme (e.g. ‘show’ and ‘examine’). This is very much a genuine example as many chemistry teachers make the same mistake in their teaching as the student makes in the question. You can see examples of this mistake being made on page 249 in one of the new text books recently published on the new programme and in the following video.

 NoS Question 1 on Energetics
NoS Question 1 on Energetics
1. The enthalpy of formation of ethanol is expressed by the following equation: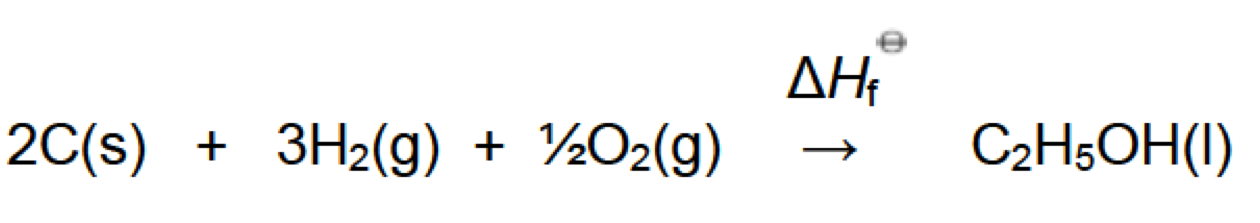
Hess’s law states that the overall enthalpy change for a reaction is independent of the reaction pathway. Based on this statement a student hypothesised that if she calculated the enthalpy of formation of ethanol using the values given for the enthalpies of combustion of graphite, hydrogen and ethanol in Section 13 of the data booklet she should get the same answer as the value given in Section 12 of the data booklet.
(a) Demonstrate that the student’s hypothesis is correct. [3]
(b) The student then reasoned that if she calculated the standard enthalpy of combustion of ethanol using the bond enthalpies given in Section 11 of the data booklet she should get an answer close to – 1367 kJ (the value given in Section 13 of the data booklet) for every mole of ethanol combusted.
She did not expect her hypothesis to be exactly correct as she realised that some of the bond enthalpies she would be using would be average bond enthalpies rather than the exact values for the bonds in ethanol.
She set out her equation showing the bonds broken and formed as follows:
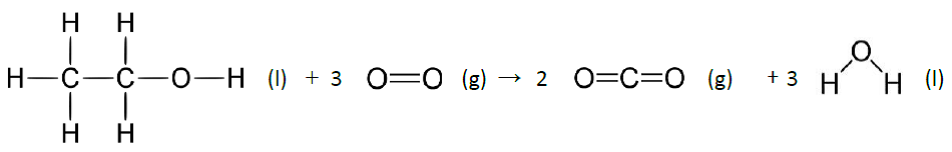
Show that the result obtained using this equation is – 1263 kJ and calculate the percentage error. [3]
(c) Examine the method used by the student in (b) and suggest how it could have been improved to obtain a more accurate result with a much smaller percentage error. [3]
To see the answers click on the 'eye'
Answers
1. (a)
.png)
By Hess’s law x = ΔH⦵f + y
x = 2ΔH⦵c(C(s)) + 3ΔH⦵c(H2(g)) = (2 x – 394) + (3 x – 286) = – 1646 kJ
y = ΔH⦵c(C2H5OH(l)) = – 1367 kJ
ΔH⦵f = x – y = – 1646 – (– 1367) = – 279 kJ
The data book value given in Section 12 is –278 kJ which is almost exactly the same. [3]
(b) Energy taken in to break bonds: = (1 x C―C) + (1 x C―O) + (1 x O―H) + (5 x C―H) + (3 x O=O)
= 346 + 358 + 463 + (5 x 414) + (3 x 498) = 4731 kJ
Energy given out to form bonds: = (4 x C=O) + (6 x O―H)
= (4 x 8041) + (6 x 463) = 5994 kJ
Since more energy is given out than taken in the reaction is exothermic.
ΔH⦵c(C2H5OH) = – (5994 – 4731) = – 1263 kJ
Percentage error = ((1367 - 1263) x 100) ÷ 1367 = 7.61 % [3]
(c). The student has not understood that bond enthalpies (and average bond enthalpies) apply to the gaseous state whereas enthalpy of combustion refers to reactants and products in their normal states at STP. She needs to include the enthalpies of vaporisation of both ethanol and water in her calculation. More energy will be evolved when gaseous water is converted into liquid water. The required value can be calculated using Section 12 and amounts to 3 x (285.8 – 241.8) = 132 kJ which brings the exothermic value up from –1263 to – 1395 kJ. However this value will be reduced by the heat that needs to be put in to vaporise the liquid ethanol to gaseous ethanol. This value is not in the data booklet but is 38.6 kJ so giving an overall value for the enthalpy of combustion of – 1356 kJ. This gives a percentage error of just 0.8% which could be accounted for by the fact that average bond enthalpies were used. [3]
1 It is worth noting that if the data book for the previous syllabus had been used the value for the C=O bond was given as 746 kJ mol-1 and using this value instead of 804 kJ mol-1 gives an answer with a considerable percentage error.

 IB Docs (2) Team
IB Docs (2) Team