Analysis of aspirin tablets
Background information
 It is an interesting fact that if you buy generic aspirin tablets they are very much cheaper than packets of branded aspirin. The analysis shows that they all usually contain the same amount of aspirin (usually 300 mg per tablet) but the branded tablets contain other ingredients so contain less aspirin in terms of percentage by mass. This practical enables students to determine the percentage of aspirin in various brands of locally available aspirin tablets. They can then work out the cost per gram of aspirin. For best results do not use soluble aspirin tablets as the other ingredients may interfere with the colour change of the indicator.
It is an interesting fact that if you buy generic aspirin tablets they are very much cheaper than packets of branded aspirin. The analysis shows that they all usually contain the same amount of aspirin (usually 300 mg per tablet) but the branded tablets contain other ingredients so contain less aspirin in terms of percentage by mass. This practical enables students to determine the percentage of aspirin in various brands of locally available aspirin tablets. They can then work out the cost per gram of aspirin. For best results do not use soluble aspirin tablets as the other ingredients may interfere with the colour change of the indicator.
I’ve put a brief history of aspirin in the student worksheet but it is worth looking at the information provided by the aspirin foundation. This gives both the history and its uses. Interestingly enough it is being trialled on 400 men in South Wales (where I live) to see if it is effective at preventing the onset of Alzheimer’s disease – but I’ve forgotten the details!! Actually you can bring in some good NOS and TOK here as there is considerable evidence that Felix Hoffmann who worked for the Bayer foundation was not in fact the first person to synthesise aspirin but just the first person to patent it so he is usually credited with the discovery.
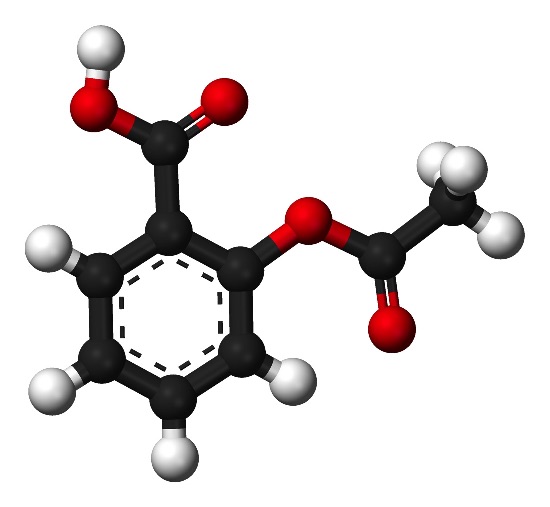 This experiment is essentially just an acid-base titration and is suitable for SL and HL. It is, of course, also suitable to cover both of the mandatory laboratory components listed in Topic 1.3 Reacting masses and volumes 'Use of the experimental method of titration to calculate the concentration of a solution by reference to a standard solution' and Topic 8.2 Properties of acids & bases. 'Candidates should have experience of acid-base titrations with different indicators'. The reaction of alcohols with carboxylic acids to form esters is covered in Topic 10.2: Functional group chemistry so even students who are not studying Option D Medicinal chemistry should have some knowledge of the chemistry of esters. All students should be able to name the phenyl, carboxyl and ester functional groups present in aspirin. For HL students you might like to mention that another way of analysing aspirin is to heat it with a known amount of excess sodium hydroxide then titrate the left over sodium hydroxide with hydrochloric acid (an example of a ‘back titration’). When heated one mol of aspirin reacts with 2 mol of sodium hydroxide as the ester is hydrolysed. You could ask them to write the equation for this reaction.
This experiment is essentially just an acid-base titration and is suitable for SL and HL. It is, of course, also suitable to cover both of the mandatory laboratory components listed in Topic 1.3 Reacting masses and volumes 'Use of the experimental method of titration to calculate the concentration of a solution by reference to a standard solution' and Topic 8.2 Properties of acids & bases. 'Candidates should have experience of acid-base titrations with different indicators'. The reaction of alcohols with carboxylic acids to form esters is covered in Topic 10.2: Functional group chemistry so even students who are not studying Option D Medicinal chemistry should have some knowledge of the chemistry of esters. All students should be able to name the phenyl, carboxyl and ester functional groups present in aspirin. For HL students you might like to mention that another way of analysing aspirin is to heat it with a known amount of excess sodium hydroxide then titrate the left over sodium hydroxide with hydrochloric acid (an example of a ‘back titration’). When heated one mol of aspirin reacts with 2 mol of sodium hydroxide as the ester is hydrolysed. You could ask them to write the equation for this reaction.
As the students pool their results you could use this experiment as an example of the use of a spreadsheet for data processing and put a number 3 in the ICT box on form 4/PSOW. It is certainly worth discussing with them why branded tables are so much more expensive. Is it just to make the shareholders of pharmaceutical companies rich?
I've included questions in the worksheet below which you could give to students to help them calculate their results.

 Student worksheet
Student worksheet
ANALYSIS OF ASPIRIN TABLETS
For a long time the bark of the willow tree (salix alba) was used as a traditional medicine to relieve the fever symptoms of malaria. In the 1860's chemists showed that the active ingredient in willow bark is salicylic acid (2-hydroxybenzoic acid) and by 1870 salicylic acid was in wide use as a pain killer (analgesic) and fever depressant (antipyretic). However, because it is a relatively strong acid, salicylic acid has the undesirable side effect of irritating and damaging the mouth, esophagus and stomach membranes. In 1899 the Bayer Company of Germany introduced the ethanoate ester of salicylic acid, naming it 'Aspirin'. Since that time mild analgesics containing aspirin have appeared under many different brand names. The aim of this experiment is to determine the percentage of aspirin present in different commercial preparations and to find which is the best value for money.
The analysis makes use of the fact that aspirin is a monoprotic (monobasic) acid and therefore reacts with sodium hydroxide according to the equation:
C6H4(OCOCH3)COOH + NaOH → C6H4(OCOCH3)COO-Na+ + H2O
ENVIRONMENTAL CARE:
None of the reactants or products is particularly harmful to the environment and the waste can be safely disposed of down the sink.
SAFETY:
No special precautions are necessary. You might like to consider why many doctors now recommend that you take paracetamol rather than aspirin for a headache even though aspirin is an effective mild analgesic.
PROCEDURE:
Note the brand name and the price of the aspirin tablets you are using. Weigh out accurately one tablet (about 0.4 - 0.5 g) into a 50 cm3 conical flask and dissolve it in 10 cm3 of 95% alcohol. Titrate with 0.100 mol dm-3 sodium hydroxide solution using two drops of phenolphthalein solution as an indicator.
CALCULATIONS:
1. What amount (in mol) of sodium hydroxide was required to react exactly with the aspirin?
2. What amount (in mol) of aspirin was present in your weighed-out tablet?
3. What is the mass of one mole of aspirin?
4. What is the percentage of aspirin in your sample?
5. Compare the mass of aspirin in the tablet that you have obtained with the value claimed by the mIB Docs (2) Teamfacturer on the side of the box. What assumptions have you made that might not be true?
6. Pool your results with others and draw out a table for the different sources of aspirin showing their percentage purity and the cost per gram of pure aspirin. Which is the best buy?
This worksheet can also be downloaded from:

 IB Docs (2) Team
IB Docs (2) Team