Boiling points of mixtures

 A practical on intermolecular forces of attraction
A practical on intermolecular forces of attraction
 This is an excellent practical and well worth doing. It introduces your students to setting up and using reflux apparatus. They have to overcome the practical problem of reading the thermometer through Quickfit apparatus (the mark always seems to be where exactly the joints are!). They have to think carefully about how to plot relevant graphs. They can check how accurate their work is by arriving at what should be the same boiling point for the 50:50 mixture by two different ways and by comparing the boiling points they obtain with literature values. In addition, it really helps them to understand the different types and strengths of intermolecular forces of attraction.
This is an excellent practical and well worth doing. It introduces your students to setting up and using reflux apparatus. They have to overcome the practical problem of reading the thermometer through Quickfit apparatus (the mark always seems to be where exactly the joints are!). They have to think carefully about how to plot relevant graphs. They can check how accurate their work is by arriving at what should be the same boiling point for the 50:50 mixture by two different ways and by comparing the boiling points they obtain with literature values. In addition, it really helps them to understand the different types and strengths of intermolecular forces of attraction.
(Teachers performing the experiment at a workshop in Buenos Aires.)
Teacher's notes
The three mixtures chosen are:
1. Propan-1-ol/propan-2-ol. This should give a straight line as the type of intermolecular forces of attraction is the same in both the pure liquids and in the mixture.

Raoult’s Law is not on the programme but this behaves as an ideal mixture due to the hydrogen bonding present throughout.

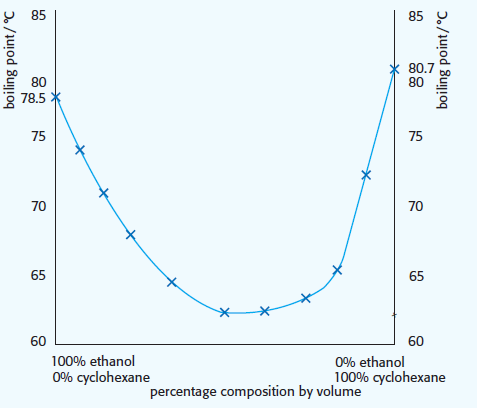
2. Cyclohexane/ethanol. The bonding in the mixture (London dispersion forces)) will be weaker than in either of the pure liquids. Ethanol has hydrogen bonding (b) and cyclohexane has London (dispersion) forces of attraction (a). The London forces of attraction in the mixture will be weaker than in the pure cyclohexane as there is less surface area overlap.
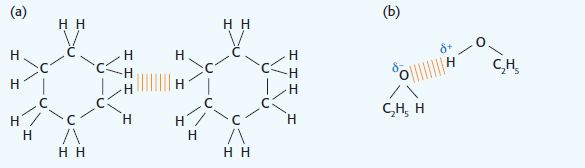
This results in the curve dipping below the boiling points of the pure substances.
3. Trichloromethane/ethyl ethanoate. This mixture has a higher boiling point than either of its components. This is because attraction can occur between the δ+ on the hydrogen of CHCl3 and the δ− on the O of CH3COOC2H5.
Traditionally the mixture used for this was trichloromethane and propanone and this gives a more pronounced curve.


In 1970 there was one isolated report of an explosion occurring (MCA Case History 1661(1970) - see reactivity profile under acetone) so ever since it has not been recommended (although I have actually used this many times in the past thirty years with absolutely no problem – do I fit the definition of an IB ‘risk taker’?!).
This experiment cover many good points. In addition to learning about different intermolecular forces of attraction students have to manipulate the data to draw the correct graph – which strictly should be boiling point against molar percentage concentration. They can calculate uncertainties and compare the values they get for the pure liquids with the literature values.

 Student worksheet
Student worksheet
BOILING POINTS OF LIQUID MIXTURES
This experiment looks at how the boiling point of a mixture of two fully miscible volatile liquids varies as the composition of the mixture alters. Three different mixtures will be investigated and you will be required to interpret the results in terms of the intermolecular attractive forces between the component liquids. The three different mixtures are:
1. C3H7OH / H3CCH(OH)CH3 (propan-1-ol/propan-2-ol)
ENVIRONMENTAL CARE:
The mixtures are all organic so pour all the residues into the container marked 'Organic Waste' in the fume cupboard. Do not pour them down the sink. There is no need to wash out the apparatus afterwards with water as it can be left to dry by evaporation.
SAFETY:
The liquids used are all volatile and, apart from trichloromethane, are highly inflammable. Avoid breathing in their vapours as much as possible and keep them well away from a naked flame. This is particularly important when adding solvent to the reflux apparatus, the Bunsen burner must first be moved to a safe distance. It is advisable to use a fume cupboard for the mixture containing trichloromethane.
PROCEDURE:

Choose one of the three mixtures 1, 2 or 3. Carefully set up the reflux apparatus as illustrated. Turn the water on into the bottom of the condenser so there is a gentle flow out from the top into the sink and check the flow from time to time. The thermometer should dip into the liquid mixture, but not touch the walls of the flask. All the heating should be done with a small blue flame as the mixtures boil easily.
QUESTIONS:
This worksheet can also be downloaded from:

 IB Docs (2) Team
IB Docs (2) Team