Hydroxychloroquine & IB Chemistry
Hydroxychloroquine has been very much in the news recently as it is a medicine that is being controversially taken by some people both as a preventative and as a treatment against Covid-19. After a brief introduction this page uses the chemistry of hydroxychloroquine holistically to test students understanding of content found in five different topics on the IB syllabus. Many of the questions can be answered by SL and HL students although some are only for HL.
 Introduction
Introduction
Hydroxychloroquine has been in the news for the past few years both as a possible preventative against Covid-19 and as a treatment for those who have already caught the virus. Currently there is no hard evidence that it is a safe or effective treatment against Covid-19. Several studies have been carried out e.g. a report in the Lancet, which has since been retracted was based on a study of 96,032 people hospitalised with Covid-19 in in 671 hospitals in six continents and concluded there was no benefit. A later study looking at the outcome of 23 such studies (although not all of them were clinically randomized) also came to the conclusion that neither hydroxychloroquine nor chloroquine have been shown to be beneficial when used for the treatment of patients with COVID-19.
Hydroxychloroquine regulates the body’s immune system and is a licensed drug used in the treatment of diseases such as malaria, rheumatoid arthritis and lupus. As well as people putting their own lives at risk from serious side effects such as dangerous heart arrhythmias and macular degeneration there is concern that people taking hydroxychloroquine as a possible preventative against Covid-19 may limit the supply of the drug to those who rely upon it for other illnesses.
Since many, if not all, of your students will have heard of hydroxychloroquine it provides an interesting subject upon which to base questions covering several of the topics covered by the IB chemistry syllabus. The questions cover stoichiometric relationships, chemical bonding and structure, acids & bases, organic chemistry and measurement & data processing. They are not designed to be exactly similar to IB examination questions but are given to demonstrate how the syllabus can be applied to a topic of current interest to stimulate your students and to increase their understanding of IB chemistry.
Although several of the questions cover the AHL programme, standard level students should be able to answer questions 1,2,3,4,7,10,11 and 12(a). Question 12(b) is of course not on the IB programme (one or two of the others sail very close to the wind!) but from the information given students should be able to deduce the answer.
Questions on hydroxychloroquine
Structure:
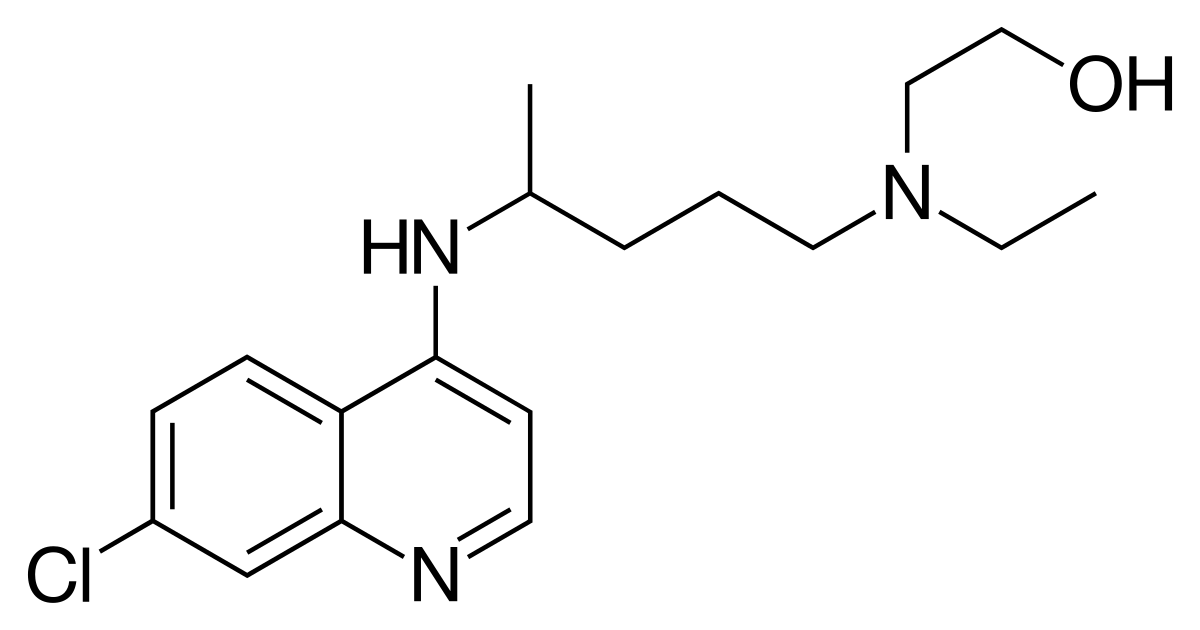
Molecular formula: C18H26ClN3O
1. Use the data given in Section 6 of the IB data booklet to calculate the relative molecular mass of hydroxychloroquine. Suggest why the value obtained is not exactly equal to the value of 335.87 given in both NIST and Chem Spider.
Using the values given for the relative atomic mass for each of the elements Mr = (18 x 12.01) + (26 x 1.01) + 35.45 + (3 x 14.01) + 16.00 = 335.92.
It is consistent to four significant figures (i.e. to 1 decimal place) but not to two decimal places. This is most probably because the values in the IB data booklet give no uncertainty as to the accuracy of the second decimal place figure. For example Ar for H is given elsewhere as 1.008. When this is multiplied by 18 it gives a value for Mr 0.036 lower than if the Ar of H is taken as 1.01.
2. Hydroxychloroquine has been licensed for use on prescription in the U.S.A. since 1955. It is sold under the brand name of Plaquenil among others as its salt with sulfuric acid, hydroxychloroquine sulfate with the molecular formula, C18H28ClN3O5S (Mr = 434). The prescribed dose of Plaquenil depends upon the weight of the patient but the tablets normally come in 200 mg or 400 mg dosages.

Calculate the mass of hydroxychloroquine present in one 400 mg tablet of its salt to three significant figures.
Percentage of hydroxychloroquine in hydroxychloroquine sulfate = (336 ÷ 434) x 100 = 77.42%
Mass of hydroxychloroquine in 400 mg of hydroxychloroquine sulfate = (400 x 77.42) ÷ 100 = 310 mg.
3. (a) Deduce the index of hydrogen deficiency (IHD) of hydroxychloroquine from its molecular formula.
The molecular formula is C18H26ClN3O. Cl atoms behave as if they are H atoms, O atoms have no effect on the IHD and N atoms can be substituted for C atoms whilst at the same time adding one to the number of H atoms so the molecular formula is equivalent to C21H30. For it to be fully saturated with an IHD of zero (i.e. CnH(2n+2)) there would need to be 44 H atoms so the IHD is (44 − 30) ÷ 2 = 7.
3. (b) Explain how the structural formula given above is consistent with the answer from 3.(a).
Except for the two ring system all the bonds in the molecule are single bonds and have an IHD of zero. The two ring system contains the equivalent of five double bonds and each ring contributes one to the IHD giving a total of seven.
4. The two ring system is known as quinoline and has the structural formula

Other than quinoline itself, identify the functional groups present in hydroxychloroquine.
Alkyl, amine, hydroxyl and chloro
5. Explain why all the electrons in the pi bonds are delocalized.
The only pi bonding in the molecule occurs in the two rings as all the other bonds in the hydroxychloroquine molecule are sigma bonds. All the nine carbon atoms and the nitrogen atom in the two fused rings are sp2 hybridized. Each carbon atom also has one electron in a p orbital above and below the plane of the molecule. The nitrogen atom also has one electron in its p orbital (in addition to the two non-bonding electrons in one of its sp2 orbitals). These ten electrons form a delocalized pi bond across all the ring atoms rather than alternate double bonds and single bonds. Hence the bonds between the carbon atoms and between the nitrogen and carbon atoms consist of one sigma bond with bond angles of 120o together with the delocalized pi bond.
6. Paquenil tablets contain a racemic mixture of the two enantiomers of hydroxychloroquine.
Identify the chiral carbon atom in hydroxychloroquine.

Chiral carbon atom shown by *
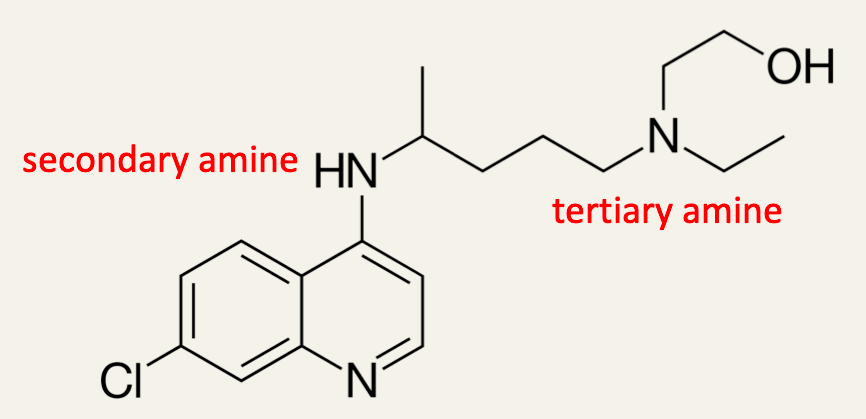
7. Identify the class of each amine present in the R− group in hydroxychloroquine.

8. Explain why the C−Cl bond length is shorter in hydroxychloroquine than the C−Cl bond in chloromethane H3C−Cl.
In chloromethane the carbon atom is sp3 hybridized and the C−Cl bond is a normal sigma bond. In the quinoline ring in hydroxychloroquine the carbon atoms and the nitrogen atom are all sp2 hybridized. A non-bonding pair of electrons in a p orbital on the chlorine atom can also combine with the electrons in the delocalized pi bond formed from the remaining p orbitals on the carbon (and nitrogen) atoms in the rings so increasing the bond strength and shortening the bond.
9. The formula of hydroxychloroquine sulfate can be written as [C18H28ClN3O]2+[SO4]2−.
Identify where the two H+ ions are located in the structure of the hydroxychloroquine cation.
Because amines are basic they will react with the sulfuric acid so that the two positive charges will be located on the two nitrogen atoms in the R− chain. The secondary amine in the chain will form N+R2H2 and the tertiary amine will form N+R3H. Although the non-bonding pair of electrons on the nitrogen atom in the quinoline group is in an sp2 orbital and does not contribute to the delocalised pi bond, quinoline is a weaker base than secondary and tertiary amines so does not act as a Lewis base in this reaction. In fact the pKb values for secondary and tertiary amines lie in the region of 3 to 4 whereas the pKb value for quinoline is 9.1 so it is a considerably weaker base (data from D.R. Lide (Ed.), CRC Handbook of Physics and Chemistry, 88th Ed, 2007). This is often explained by stating that a non-bonding pair of electrons in an sp2 hybridized orbital are closer to the N nucleus (so less attractive to a proton) than if they are in an sp3 hybridized orbital as the percentage of s orbital in the hybridization is greater.
10. Hydroxychloroquine can be synthesised in several different ways. One synthesis by R.S. Vardanyan and V.J. Hruby in 2006 occurs in three separate steps. The first step involves the reaction of Compound A with 2-ethylaminoethanol to form an intermediate (Intermediate 1).

(a). Name Compound A
5-chloro-2-pentanone (since the IB only requires you to be able to name compounds containing just one functional group other acceptable answers are 5-chloro-pentan-2-one, 1-chloro-4-pentanone and 1-chloropentan-4-one).
(b). Describe the type of reaction that occurs in Step 1.
Nucleophilic substitution. This is covered in 10.2 on the IB syllabus although the only nucleophile referred to there is the hydroxide ion. Ammonia and amines also possess a non-bonding pair of electrons so are good nucleophiles. Because the chlorine atom is on the end carbon atom the mechanism is likely to be SN2 as it is a primary halogenoalkane.
11. In the second step the intermediate from Step 1 is reacted with a mixture of hydrogen and ammonia to form a second intermediate in which the carbonyl group has been replaced by an amine group.
.png)
What is the function of (a) the hydrogen and (b) the solid nickel?
(a) The hydrogen is acting as a reducing agent (or hydrogenating agent) and (b) the solid nickel is acting as a heterogeneous catalyst.
12. In the third and final step the intermediate from Step 2 is reacted with 4,7-dichloroquinoline to form hydroxychloroquine.
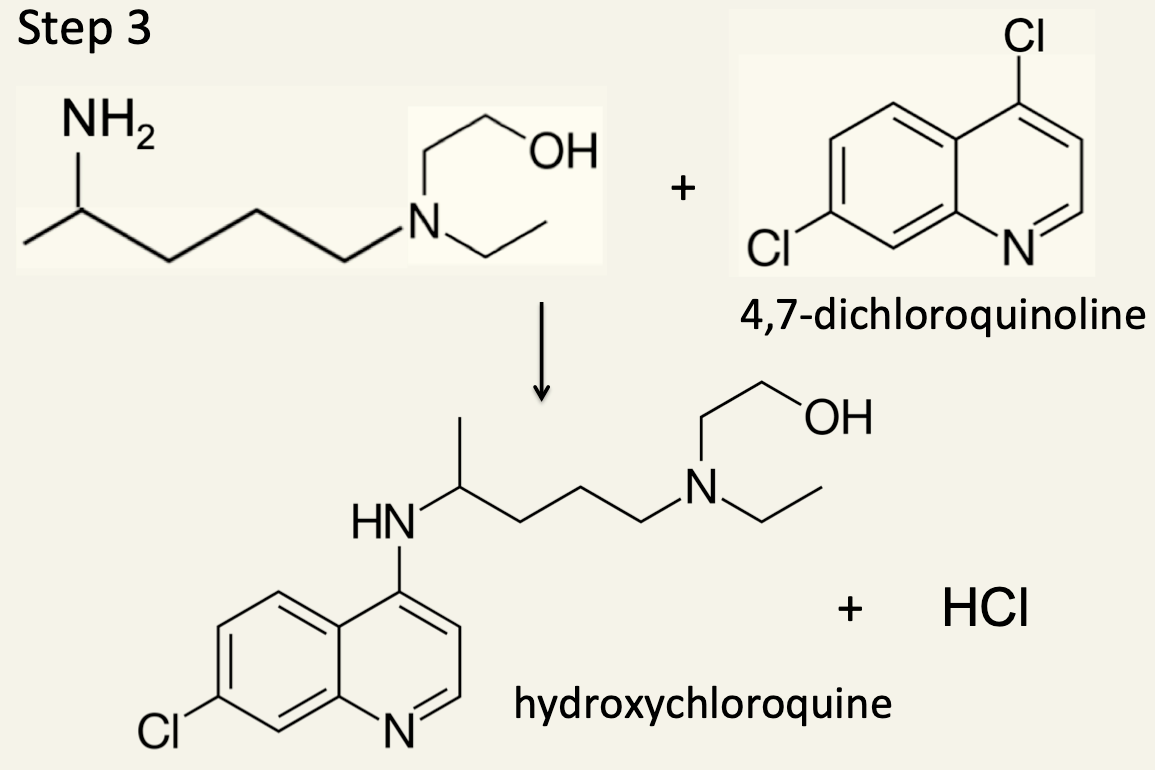
(a) Identify the type of reaction taking place.
This is another example of a nucleophilic substitution reaction. The nucleophile is a primary amine whereas in Step 1 the nucleophile is a secondary amine.
(b) Using information from Step 3 name the following compound
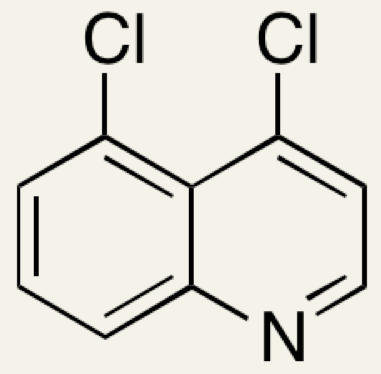
4,5-dichloroquinoline. The numbering system starts from the N atom. From the given structure of 4,7-dichloroquinoline it can be seen that although there are a total of nine carbon atoms in the two rings, the two C atoms where the rings join are saturated with other carbon atoms and cannot bond to any other atoms without destroying the delocalization of the ring so do not count in the numbering system.


 IB Docs (2) Team
IB Docs (2) Team