Covalent structures (2)

 4.3 &
4.3 &  14.1 Covalent structures (2) (2 hours)
14.1 Covalent structures (2) (2 hours)
Geometry of simple molecules & ions
This is the second part of 4.3 Covalent structures and looks at VSEPR theory to determine the shapes of simple molecules and ions. Since the underlying theory is the same it also includes 5 and 6 electrons pairs around the central atom which is on the Higher Level programme under 14.1.
Pause for thought
Part of sub-topic 4.3 is to predict the bond angles in species with two, three and four electrons domains. The classic example used for both Standard and Higher Level students is to compare the bond angles in methane, ammonia and water. It all seems highly logical. In methane all the four pairs of electrons (electron domains) are bonding pairs so repel each other equally to give a regular tetrahedron with bond angles of 109.5o. Ammonia contains one non-bonding pair which repels bonding pairs more than other bonding pairs so the bond angle decreases to about 108o in the trigonal pyramid molecule. The two non-bonding pairs in water cause even more repulsion so the bond angle in the bent molecule decreases further to about 104.5o. This can be found in all the text books and the theory perfectly matches the experimentally found bond angles.
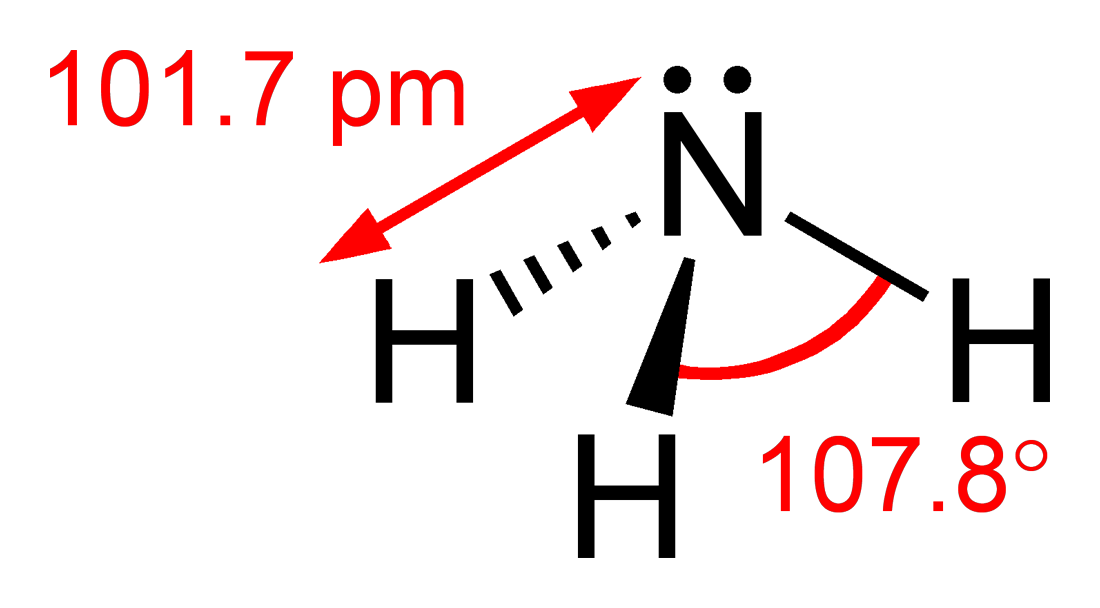

However this is yet another case of where the IB (and other pre-university courses) conveniently does not explore further as the logic appears flawed. Consider applying the same logic to phosphorus trichloride. Like ammonia there are three bonding pairs and only one non-bonding pair so one would expect the bond angle to be just less than 109.5o and more than 104.5o. In fact the bond angle is 100o. You might argue that it is the chlorine that perhaps makes the difference. So let's look at phosphine where the only difference with ammonia is that a phosphorus atom has replaced the nitrogen atom. The bond angle of 93.5o is even lower than phosphorus trichloride and considerably lower than the H-O-H bond angle in water where there are two non-bonding pairs.


An astute student might argue that the bond angle also depends upon the period where the central atom is located since nitrogen and oxygen are in period 2 and phosphorus is in period 3. There is some truth in this. What would you expect the bond angle in hydrogen sulfide to be? On the simple theory it might be expected to be similar to water i.e. about 104.5o. If we now use what we know about phosphine then we might expect it to be a little less than 93.5o. Guess what - it is 92.1o - our new theory works! We could extend this and try to predict the bond angle for sulfur dichloride. Using our new logic we might expect it to be perhaps one or two degrees less than the 100o in phosphorus trichloride as there are now two non-bonding pairs of electrons? Wrong! It is 103o.
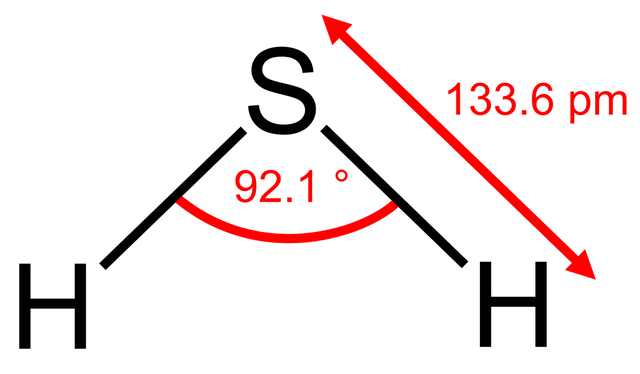
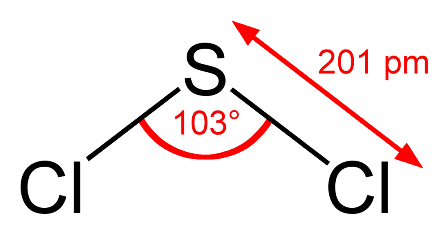
Clearly it is not nearly as simple as it is made out. Can we really expect students to predict these bond angles so that they agree with the experimentally determined values?
I don't think so!!
Nature of Science
Models are used by scientists as representations of the real world—VSEPR (the model of the shapes of simple molecules and ions) has been developed to explain observable properties.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesThe term “electron domain” will be used in place of “negative charge centre”. Although not specifically listed on the programme suitable examples might include CH4, NH3, H2O, NH4+, H3O+, BF3, C2H4, SO2, C2H2 and CO2.
International-mindedness(Nothing is listed in the programme for international-mindedness under this sub-topic) |
Teaching tipsYou can get students to arrive at shapes by considering the hybridization of the central atom. However as hybridization is not on the syllabus for Standard Level and as d2sp3 hybridization is not on the syllabus for Higher Level it is best not to go down this path except perhaps for carbon compounds with Higher Level students. In fact VSEPR theory is very simple to explain (even though students seem to find it difficult - see Paper 2: Areas of difficulty VSEPR). Electron pairs (domains) try to arrange themselves so that they are as far away from each other as possible. This leads to the basic shapes for 2,3,4,5 and 6 pairs. To find the actual shape there are really only three points to consider: I then work through the possibilities with some of the classic examples: Then explain why the H-O-H bond angle for H2O is smaller than H-N-H bond angle in NH3 which in turn is smaller than H-C-H bond angle in CH4
Throughout all of this get them to make actual three dimensional models of each shape that they can hold in their hands (rather than just see on a two-dimensional screen) and explain bond angles and how the 3-D shapes can be drawn (represented) in two-dimensions. Once the shapes are known molecular polarities are relatively straightforward. One problem you might encounter with five pairs is how to name the shape formed when there are four bonding pairs and one non-bonding pair. This is the case for sulfur tetrafluoride, SF4. I have variously seen K-shaped, distorted tetrahedral or see-saw. See-saw is the name given in Wikipedia. | Study guide
Page 27 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MC test: Covalent structures (2). For short-answer questions which can be set as an assignment for a test, homework or given for self study together with model answers see Shapes & polarity questions.Vocabulary listelectron domain IM, TOK, 'Utilization' etc.See separate page which covers all of Topic 4 & 14. Practical work
|
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A rather nice animation of shapes by a student - Ashley Jennings
2. A tutorial by Joey Smokey from Clark College Tutoring and Writing Center. This is just a lecture but the diagrams of the shapes are very clear.

 IB Docs (2) Team
IB Docs (2) Team 

































