Covalent structure (2) Shapes & polarity questions
![]()
![]() Questions on Topic 4.3: Shapes & polarity
Questions on Topic 4.3: Shapes & polarity
Note that the last two questions are for ![]() only.
only.
Predict the shape and bond angles of:
i. boron trichloride, BCl3
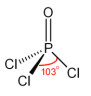
ii. phosphoryl chloride, POCl3
iii. phosphine, PH3
iv. hydrogen cyanide, HCN
Explain why sulfur dioxide molecules, SO2, have a bent shape whereas carbon dioxide molecules, CO2, are linear
Explain why C=O bonds are polar and yet the carbon dioxide molecule is non-polar.
Explain why the H-N-H angle in ammonia is smaller than the H-N-H angle in the ammonium ion.
A simplified model of benzene, C6H6, shows the six carbon atoms in a ring with alternate single and double bonds between the carbon atoms. Each carbon atom is also bonded to one hydrogen atom.
i. Based on the model described above predict the  bond angle in benzene.
bond angle in benzene.
ii. In cyclohexane, C6H12 the six carbon atoms are also in a ring but are joined to each other only by single bonds. Each carbon atom is also bonded to two hydrogen atoms. Predict the  bond angle in cyclohexane.
bond angle in cyclohexane.
Fluorine and oxygen are very electronegative elements. Explain why hydrogen fluoride, HF, and water, H2O, are very polar molecules but tetrafluoromethane, CF4, and carbon dioxide, CO2 are non- polar.
![]() only
only
Predict the shape of:
i. xenon tetrafluoride, XeF4
ii. the iodine tetrachloride ion, ICl4–
iii. chlorine trifluoride, ClF3
![]() only
only
Predict all the  bond angles in:
bond angles in:
i. phosphorus pentafluoride, PF5
ii. the phosphorus hexafluoride ion, PF6–

 IB Docs (2) Team
IB Docs (2) Team 
 It contains 5 electron pairs (3 bonding + 2 non-bonding) so predicted to be T-shaped
It contains 5 electron pairs (3 bonding + 2 non-bonding) so predicted to be T-shaped