Fundamentals of organic chemistry (2) questions
 Assignment:
Assignment: 
 Questions on Topic 10.1 : Fundamentals of organic chemistry (2)
Questions on Topic 10.1 : Fundamentals of organic chemistry (2)
This page of questions can be marked as direct student access either for assigning as a test or for students to work on in their own time. If you do not wish to use student access, links to downloadable versions of the questions and, separately the worked answers, can be found at Printable versions of written tasks.
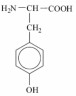
State the condensed structural formulas, together with their IUPAC names, for all the structural isomers of C6H14.
Models showing the structural formulas of two compounds A and B, both with a similar molar mass (74 and 72 g mol−1 respectively), are shown below:

i. Identify the functional groups contained in compounds A and B.
ii. State the IUPAC names for compounds A and B.
iii. Deduce which of the two compounds, A or B, will have the higher boiling point and which will be more soluble in water. Explain your deduction.
(a) State the IUPAC names of the following three alcohols:

(b) Classify these three alcohols in terms of primary, secondary or tertiary.

 IB Docs (2) Team
IB Docs (2) Team 