Oxidation & reduction

 9.1 Oxidation & reduction (4 hours)
9.1 Oxidation & reduction (4 hours)
Pause for thought
1. Definitions of oxidationWe should encourage students to think critically and ask searching questions. In the previous syllabus this topic started with the definition that oxidation is the loss of electrons. In this new programme it states "Oxidation and reduction can be considered in terms of oxygen gain/hydrogen loss, electron transfer or change in oxidation number". It is worth examining this statement.
The combustion of hydrogen that occurred during the Hindenburg zeppelin airship disaster in 1937 killed 35 people. It was clearly an example of oxidation as oxygen added to hydrogen to form water.
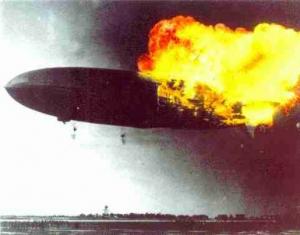
In fact two of the classic examples of oxidation are the combustion of hydrogen to form water and the combustion of carbon to form carbon dioxide. Much of the energy we use in the World comes from burning a combination of these two elements in the form of hydrocarbons. Many text books do still define oxidation in terms of electron loss and reduction in terms of electron gain but are any electrons actually transferred when hydrogen burns in oxygen to form water or when carbon or hydrocarbons burn?
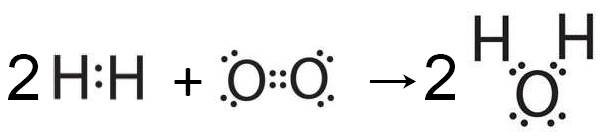
In all of these examples it is obvious that the hydrogen and the carbon are oxidized as oxygen is added and yet in none of these cases does the substance being oxidized lose any electrons. Students already know from sub-topic 4.2 : Covalent bonding that each hydrogen atom shares two electrons in hydrogen gas, hydrocarbons and in water, the oxidized product. Similarly each carbon atom shares eight electrons in graphite and eight electrons in hydrocarbons and in carbon dioxide, the oxidized product, it still shares eight electrons. They should understand that although the loss of electrons is always oxidation the converse is not always true. You can read more about this in the TOK section in Topics 9 & 19.
2. Why is lithium above potassium in the activity series?
The activity series for students to use is given in Section 25 of the IB data booklet. Students and teachers know that potassium reacts more vigorously than lithium when it is added to water. so why is lithium listed above potassium in the activity series? For a full explanation of this and how the activities series for metals differs from the reactivity series for metals see the separate page on Understanding the activity series.
3. A practical example.
The syllabus now includes one very specific environmental application of redox processes - the determination of dissolved oxygen in water. In fact the syllabus goes slightly further than this as it relates the amount of dissolved oxygen to the biochemical oxygen demand (BOD). Although this provides a good example to test whether students can apply redox equations when performing calculations it is rather out of date as modern methods to determine BOD bypass this procedure and simply use an oxygen probe (sensor) connected to a data logger (see other resources 3 below).
 The fact that oxygen dissolves in water is essential for aquatic life. The solubility of oxygen in water depends on several factors with the two most important ones being the salinity of the water and the temperature – an increase in both lowers the amount of dissolved oxygen. The amount of dissolved oxygen present also depends upon the amount of algae or organic material in the water both of which use up oxygen. Biochemical oxygen demand (BOD) is a measure of the amount of dissolved oxygen required to decompose organic matter in water at a fixed temperature over a certain time period (usually quoted at 20 oC over five days). It is a useful measure to determine the degree of pollution of a water sample. Water with a BOD of more than 5 ppm is generally considered polluted. Even water with a high BOD can still recover and be reoxygenated if the water is fast flowing.
The fact that oxygen dissolves in water is essential for aquatic life. The solubility of oxygen in water depends on several factors with the two most important ones being the salinity of the water and the temperature – an increase in both lowers the amount of dissolved oxygen. The amount of dissolved oxygen present also depends upon the amount of algae or organic material in the water both of which use up oxygen. Biochemical oxygen demand (BOD) is a measure of the amount of dissolved oxygen required to decompose organic matter in water at a fixed temperature over a certain time period (usually quoted at 20 oC over five days). It is a useful measure to determine the degree of pollution of a water sample. Water with a BOD of more than 5 ppm is generally considered polluted. Even water with a high BOD can still recover and be reoxygenated if the water is fast flowing.
The amount of dissolved oxygen is traditionally determined using the Winkler method. This was devised by the Hungarian Lajos Winkler (1863-1939) for his Ph.D. thesis in 1888. Excess manganese(II) sulfate and hydroxide ions are added to a known volume of the water sample. The dissolved oxygen oxidises the manganese(II) ions to give a brown precipitate (usually considered to be a hydrated form of manganese(IV) oxide). In the presence of acid this then oxidises iodide ions into iodine and is itself reduced back to manganese(II). The iodine produced is titrated with standard sodium thiosulfate solution using starch as the indicator.
2Mn2+(aq) + O2(aq) + 4OH− (aq) → 2MnO2(s) + 2H2O(l)
(Note that some texts give the formula of the Mn(IV) product as MnO(OH)2 but this does not affect the overall stoichiometry.)
MnO2(s) + 2I−(aq) + 4H+(aq)→ Mn2+(aq) + I2(aq) + 2H2O(l)
2S2O32−(aq) + I2(aq) → S4O62−(aq) + 2I−(aq)
By following the stoichiometry it can be seen that one mole of dissolved oxygen is equivalent to four moles of thiosulfate ions.
Nature of science
Scientists often broaden similarities to general principles. The way in which the definition of redox has changed from one involving just oxygen and hydrogen to one involving the transfer of electrons and a change in oxidation states is a good example of this.
Learning outcomesAfter studying this topic students should be able to: Understand
Apply their knowledge to:
| Clarification notesAlthough oxidation number and oxidation state are often used interchangeably IUPAC does formally distinguish between the two terms. According to IUPAC oxidation numbers are represented by Roman numerals and oxidation states should be represented with the sign given before the number, e.g. +3 and not 3+. The oxidation state of hydrogen (-1) in metal hydrides and oxygen (-1) in peroxides should be known. An activity series is given in Section 25 of the data booklet. International-mindednessThe United Nations recognises access to a supply of clean drinking water as a fundamental human right. Even so, over one billion people in the world are thought to lack this basic provision. Oxidizing agents, such as chlorine or ozone, are commonly used to disinfect water by killing microbial pathogens. |
Teaching tipsI normally introduce this by building up from the basic definition that oxidation is the addition of oxygen and then show how this definition has been extended to include the loss of hydrogen and then the loss of electrons. Similarly reduction is the loss of oxygen, gain of hydrogen or the gain of electrons. Using an example such as the combustion of magnesium in air they can easily deduce that magnesium loses electrons and oxygen gains electrons. I'd rather they used examples like this to understand the definitions but many will still use OILRIG as an aide memoire. For oxidation states you will need to first give them the 'rules'. These can be found in most text books but it is worth stressing that they are just a useful tool that chemists have devised as they are based on the false assumption that covalent compounds can be assumed to be ionic. I then give them a whole list of compounds and ask them to deduce the oxidation state of each element. Once they 'get' it I then give a series of reactions where they can see that the reaction is only a redox reaction if there is a change in oxidation states. Oxidation is an increase in oxidation state and reduction is a decrease in oxidation state. You will need to carefully explain the difference between oxidation state, oxidation number, charge on an ion and charge of a complex ion, i.e. using +2, (II) and +2 and 2+ in a compound such as CuSO4(aq). Although the current syllabus emphasises this, it still mixes oxidation number and oxidation state up in various places (for example, the data booklet in Section 14 talks about oxidation numbers and then lists oxidation states!). Be careful with past examination questions too. In the past (II) has not been accepted as an answer for the oxidation number of Cu in CuSO4 - the answer they wanted was +2. Now (II) would appear to be the only correct answer!! | Study guide
Pages 70 - 72 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MC test: Oxidation & reduction. For three different sets of short-answer questions which can be set as an assignment for a test, homework or given for self study together with model answers see Oxidation & reduction questions (1), Oxidation & reduction questions (2) and Oxidation & reduction questions (3). Vocabulary list:Oxidation IM, TOK, 'Utilization' etc.See separate page which covers all of Topic 9. Practical work |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A video that shows how oxidation states change across the periodic table (although the terms oxidation states and numbers are interchanged at times).
2. Deducing whether an element has been oxidized or reduced during a redox reaction using oxidation states (note that the video uses oxidation numbers rather than states as this was from the old programme).
3. The old fashioned Winkler method may be good but it is much easier these days to use a dissolved oxygen probe. The video shows how easy it is to use the Nexsens dissolved oxygen sensor.

 IB Docs (2) Team
IB Docs (2) Team 

































