Topics 2 & 12
2 (
 6 h) & 12 (
6 h) & 12 ( 2 h): Atomic structure
2 h): Atomic structure
Some comments
A good understanding of Atomic structure is paramount to understanding chemistry. Chemical reactions involve the rearrangement of valence electrons so understanding the structure of an atom and the nature and behaviour of electrons is clearly important. This topic also brings in much Nature of Science and Theory of Knowledge as it concerns particles that are too small to ever be seen directly and provides good examples of how theories and paradigms have changed over the years. An initial glance at the syllabus is encouraging. The work of scientists like Rutherford and Thomson are referred to under ‘Nature of Science’. The ‘Aims’ include references to simulations of the gold foil experiment and use of discharge tubes.
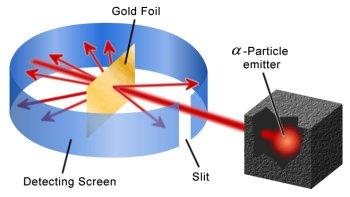
An illustration of the Geiger and Marsden experiment to show that an atom is largely empty space.
‘Utilization’ includes reference to the use of radioactive isotopes in medicine and geological dating and reference to atomic absorption spectroscopy. It also includes PET (positron emission tomography) and its use to detect cancers. In addition CERN appears under ‘International mindedness’ and Heisenberg’s Uncertainty Principle appears under ‘Theory of Knowledge’. All of this is heady stuff and appears in the two sub-topics of the core so applies to Standard Level as well as Higher Level. However this topic also clearly illustrates the weaknesses as well as the strengths of the new programme. The amount of time given to cover this topic is just 6 hours. This is clearly insufficient to cover all of the above. This is recognised by the fact that what will be examined is essentially only what is listed under ‘Understandings’, Applications and skills’ and ‘Guidance’ (with possibly a little of what is listed under 'Nature of Science'). It is here that the examinable content does not match up to material listed elsewhere. There is one big (and welcome) improvement to the new programme compared to previous guides and that is that Standard Level students are now required to know that energy levels can be split into s, p, d and f sub-levels. This is helpful when it comes to explaining several important concepts in chemistry. For example, the layout of the periodic table into blocks including lanthanoids and actinoids (3.1) and why benzene undergoes substitution rather than addition reactions (10.2). However there seems to be no actual requirement for students to have any understanding of the underlying theory or evidence. In the past Standard level students needed to be able to explain the shape of the graph obtained by plotting first ionization energies against atomic number (see right). 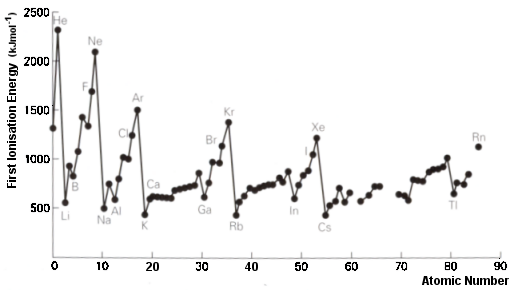 This graph provides good evidence for sub-levels and yet strangely this explanation in terms of main energy levels and sub-levels is now only specifically required by Higher Level students (see 12.1) although in the 'Guidance' for Topic 3.2 it does say rather vaguely 'For ionization energy the discontinuities in the increase across a period should be covered'. Similarly Standard Level students now need to be able to deduce the electron configuration of elements and ions including the anomalies of chromium and copper using the aufbau principle, Hund’s rules and the Pauli exclusion principle and yet they do not need to be able to state these rules or principles specifically, let alone understand them in terms of quantum theory. There is no mention of the evidence for the existence of sub-atomic particles or how their mass and charge can be measured in the examinable material and in fact it states that the operating principles of a mass spectrometer are not required. No understanding of alpha, beta and gamma radiation is mentioned which is required to understand radioisotopes and since the syllabus states that specific examples of isotopes need not be learned presumably there is no real incentive to include radioisotopes. In many ways the examinable content of what is written seems at odds with good pedagogy. According to the IB Learner Profile, students are supposed to show curiosity and engage in critical thinking. Surely we want to encourage our students to ask questions like ‘how do we know this information? Six hours is not a lot of time to cover this topic. As a teacher you will need to decide how deep to go and what examples to cover. Do you simply follow what is on the examinable programme and tell your students to accept it without question or do you encourage them to think critically and provide them with the resources to go at least some way towards explaining the facts? It will depend to some extent on the amount of time you have and on the ability and motivation of your students. I think at the very least students should be made aware of the work of scientists like Thomson, Rutherford, Geiger, Marsden, Mosley and Chadwick and have some idea of how, for example, carbon dating works. I would also use the graph of first ionization energies against atomic number as evidence for the existence of sub-levels. I would also make some reference to Heisenberg and Schrödinger to at least give a little background to the shapes of s and p orbitals and also briefly mention quantum theory to help explain electron configurations.
This graph provides good evidence for sub-levels and yet strangely this explanation in terms of main energy levels and sub-levels is now only specifically required by Higher Level students (see 12.1) although in the 'Guidance' for Topic 3.2 it does say rather vaguely 'For ionization energy the discontinuities in the increase across a period should be covered'. Similarly Standard Level students now need to be able to deduce the electron configuration of elements and ions including the anomalies of chromium and copper using the aufbau principle, Hund’s rules and the Pauli exclusion principle and yet they do not need to be able to state these rules or principles specifically, let alone understand them in terms of quantum theory. There is no mention of the evidence for the existence of sub-atomic particles or how their mass and charge can be measured in the examinable material and in fact it states that the operating principles of a mass spectrometer are not required. No understanding of alpha, beta and gamma radiation is mentioned which is required to understand radioisotopes and since the syllabus states that specific examples of isotopes need not be learned presumably there is no real incentive to include radioisotopes. In many ways the examinable content of what is written seems at odds with good pedagogy. According to the IB Learner Profile, students are supposed to show curiosity and engage in critical thinking. Surely we want to encourage our students to ask questions like ‘how do we know this information? Six hours is not a lot of time to cover this topic. As a teacher you will need to decide how deep to go and what examples to cover. Do you simply follow what is on the examinable programme and tell your students to accept it without question or do you encourage them to think critically and provide them with the resources to go at least some way towards explaining the facts? It will depend to some extent on the amount of time you have and on the ability and motivation of your students. I think at the very least students should be made aware of the work of scientists like Thomson, Rutherford, Geiger, Marsden, Mosley and Chadwick and have some idea of how, for example, carbon dating works. I would also use the graph of first ionization energies against atomic number as evidence for the existence of sub-levels. I would also make some reference to Heisenberg and Schrödinger to at least give a little background to the shapes of s and p orbitals and also briefly mention quantum theory to help explain electron configurations.
The links on the left give you teaching tips etc. for each of the sub-topics together with questions and answers for each sub-topic.
Once you have finished teaching the whole topic you can give the multiple choice tests on Atomic structure (together with answers):

 IB Docs (2) Team
IB Docs (2) Team