Metallic bonding

 4.5 Metallic bonding (0.5 hours)
4.5 Metallic bonding (0.5 hours)
Pause for thought
The not so rare ‘rare earth elements’
 Sub-topic 4.5 is very straightforward and it clearly states the trends should be limited to s and p block metals. As I point out in the teaching tips below there is a problem with the p block metal trends in group 14 as although tin has a smaller atomic radius than lead (1.40 x 10-10 m compared to 1.45 x 10-10 m according to the IB data booklet) it has a lower melting point (232 oC compared to 328 oC).
Sub-topic 4.5 is very straightforward and it clearly states the trends should be limited to s and p block metals. As I point out in the teaching tips below there is a problem with the p block metal trends in group 14 as although tin has a smaller atomic radius than lead (1.40 x 10-10 m compared to 1.45 x 10-10 m according to the IB data booklet) it has a lower melting point (232 oC compared to 328 oC).
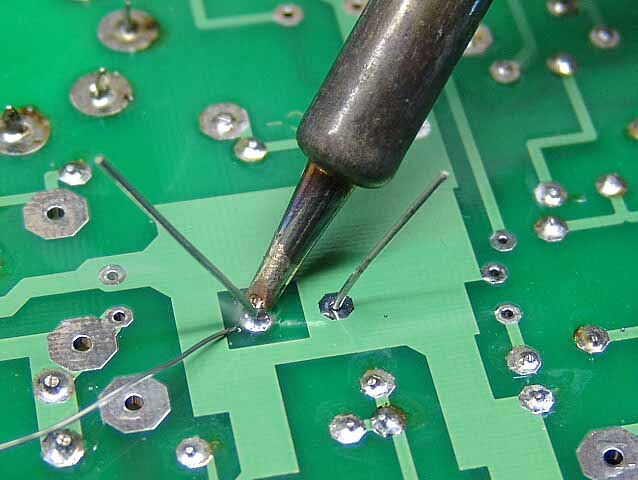
Although tin and lead form many different alloys, e.g. solder (shown being used in a circuit board in the image on the right), many of the best alloys come from combinations of transition metals. This is because transition elements in the same period have very similar atomic radii so inserting different metals into the crystal structure of a metal to form an alloy does not significantly disrupt the structure. For example, stainless steel contains iron (atomic radius 1.24 x 10-10 m) and up to 13% chromium (atomic radius 1.30 x 10-10 m) and may also contain nickel (1.17 x 10-10 m) and manganese (1.29 x 10-10 m).
The international-mindedness part of the syllabus refers to national wealth being related to the availability of metal resources, and the means to extract them. In sub-topic 3.1 The periodic table the syllabus also refers to the lanthanoids and actinoids. In recent years there has been much investment in extracting and purifying the so called rare earth metals. These are essentially fifteen lanthanoids together with scandium and yttrium. In fact they are not particularly rare, for example cerium has a similar abundance in the Earth’s crust to copper. However they do have many uses in modern technology.
.png)
Rare earth metals can be difficult and costly to mine and extract from their ores as they are often associated with radioactive material. In the 1990s the Chinese undercut many other sources and China is today the main supplier of rare earth elements. However in the past few years the Chinese have restricted the amount they export. This has caused the price of rare earth metals to rise and made the rest of the world seek new and alternative sources and also reopen mines that they had previously closed.
Nature of science
Natural phenomena can be explained by theories. For example, metals have different properties to covalent and ionic compounds because the "sea" of delocalized electrons forms non-directional bonds.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesThe trends should be limited to s- and p-block elements. Various alloys should be covered (although the syllabus does not list which actual examples should be included). International-mindednessOne factor in determining national wealth is the availability of metal resources, and the means to extract them. This varies greatly from country to country. The demands for different metals change as technologies develop and in order to manage the supply of these finite resources careful strategies are required. |
Teaching tipsMetallic bonding is treated very simply by the IB. All students really need to know is that the outer electrons become delocalized so that a metal essentially consists of an array of positive ions (cations) within a 'sea' of delocalized electrons. Because the electrons are free to move metals are good electrical conductors. Since the electrons are mobile they are also good conductors of kinetic energy in the form of heat. Generally the smaller and more highly charged the cation then the greater the attraction to the electrons and the higher the melting point although it does also depend upon the way in which the metal atoms are packed. This trend holds true for descending group 1 (the alkali metals) but it is worth pointing out to your students that it is not true within group 14 as tin (m.pt 232 oC) has a lower melting point than lead (m.pt 328 oC) even though it has a smaller ionic radius. Similarly magnesium has a lower melting point than the other metals in group 2 which do then follow the trend. Examples of alloys you should probably cover include steel (including stainless steel), bronze, brass, amalgams and maybe also solder and pewter. Because the added metals are likely to have a different radius and may also have a different charge they distort the structure of the original metal as the bonding is less directional. Because of this alloys may have lower melting points than their component metals. | Study guide
Pages 30 & 31 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MC test: Metallic bonding. For short-answer questions which can be set as an assignment for a test, homework or given for self study together with model answers see Metallic bonding questions. Vocabulary listalloy IM, TOK, 'Utilization' etc.See separate page which covers all of Topic 4 |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A video made by Richard Thornley specifically for this topic.
2. A fairly simplified video from two teachers, Jonathan Bergman and Aaron Sams which covers the basics of metallic bonding and why they conduct heat and electricity and are malleable.
3. Another video (for A level) by SnapRevise relating the properties of metals to metallic bonding.

 IB Docs (2) Team
IB Docs (2) Team 















