NOS & Critical Thinking
In order to think critically in chemistry a knowledge of both chemistry and the nature of science is required. This page gives a simple example of this then provides a full worked example you can work through. It will show you that even if the assumptions you make to arrive at a prediction give the correct prediction it does not follow that the assumptions you used are correct.
Introduction
.png) Critical thinking cannot take place in a vacuum. In order to be able to think critically about chemistry a good knowledge and understanding of chemistry and its underlying culture, i.e. the nature of science, is required. A good student should be able to question the source and accuracy of their knowledge and the way in which they use it.
Critical thinking cannot take place in a vacuum. In order to be able to think critically about chemistry a good knowledge and understanding of chemistry and its underlying culture, i.e. the nature of science, is required. A good student should be able to question the source and accuracy of their knowledge and the way in which they use it.
Some years ago I wrote a blog titled “104Ku - an anachronism in a gulag” about a visit I made to the site of Karlag, one of the former Soviet gulags in what is now Kazakhstan. In the museum I found a room with chemical apparatus and a periodic table which was said to have been used by some of the scientists who had been prisoners there during the Stalinist period.

Mendeleev periodic table in the museum at Karlag, a former Soviet gulag
Note that the ‘periodic table’ is called the Mendeleev table, the name by which it is still known in the former Soviet Union countries. The periodic table shows one of the elements having the symbol Ku.
Only a scientist who is familiar with the elements and the way in which they can be arranged in a periodic table might question the symbol Ku for element 104, as the accepted symbol is Rf. This led me to research the origin of element 104. It was first discovered in 1964 by scientists working at the Joint Institute of Soviet Research in Dubna in the USSR by bombarding plutonium-242 with neon-22. The Soviet scientists named it kurchatovium with the symbol Ku after Igor Kurchatov (1903-1960) who was the “father of the Soviet atomic bomb”. In 1969 element 104 was independently synthesised at the University of California, Berkeley by bombarding californium-249 with carbon-12. The Americans claimed that they published their results first and that their work was the first to be verified independently and so claimed the right to name the element and proposed the name rutherfordium (Rf) after Ernest Rutherford (1871 – 1937). It took until 1997 before IUPAC accepted the name of the element officially as rutherfordium. What this research does show is that the Mendeleev table in the museum cannot have been in use at the gulag as the last prisoners left the gulag in 1959 – five years before kurchatovium was first discovered.
This story illustrates the need to have some prior knowledge to be able to think critically. One good source of examples where critical thinking is necessary is either past examination questions or practice IB questions that can be found on the Internet where the chemistry required to solve the question has not really been thought through properly. The following question is one such example. Since most students learn about the energetics part of the syllabus after they have been taught periodicity, Higher Level students should have the necessary prior knowledge to answer the question critically.
Question
Predict whether the hydration enthalpy of Fe2+ ions will be more or less exothermic than the hydration enthalpy of Mn2+ ions.
Some hydration enthalpies of ions are given in Section 20 of the IB data booklet but the values for Fe2+ and Mn2+ are not included so you might use your prior knowledge of IB chemistry together with the accompanying logic to answer this question in the following way.
First you need to recall that hydration energy involves the reaction converting the gaseous M2+(g) ion into the hydrated ion M2+(aq). The smaller the ion and the more highly charged the ion the greater the charge density and the greater the attraction to water molecules and hence the more exothermic the hydration enthalpy.
Both Fe2+ and Mn2+ ions have the same charge of 2+ so their relative charge densities will depend upon their radii. The electron configurations are [Ar]3d6 for Fe2+ and [Ar]3d5 for Mn2+. As Z = 26 for Fe and Z = 25 for Mn, Fe2+ ions have one more proton in their nucleus acting on electrons in the same outer energy level so will have a smaller radius than Mn2+ ions. This means that Fe2+ ions will have the greater charge density so the enthalpy of hydration will be predicted to be more exothermic.
This answer agrees with the actual data as the values for the hydration energies of Fe2+ and Mn2+ are − 1950 and – 1851 kJ mol−1 respectively (see table at foot of page). This deduction and prediction seems fine but supposing the question had asked you to predict which of Fe2+ ions or Co2+ ions is more likely to have the greater exothermic enthalpy of hydration. Both have the same charge of 2+, the electron configurations for Fe2+ and Co2+ are [Ar]3d6 and [Ar]3d7 respectively and the atomic numbers of Fe and Co are 26 and 27 respectively. Following the same logic, you can predict that Co2+ has a smaller ionic radius than Fe2+ and the enthalpy of hydration of Co2+ will be greater than Fe2+ as it has a greater charge density. This again agrees with the hydration enthalpy data as the value for Co2+ is − 2010 kJ mol−1 which is more exothermic than the value of − 1950 kJ mol−1 for Fe2+. This too all looks fine and it seems reasonable for you to assume that as your predictions are correct the logic and knowledge used to arrive at them must also be correct.
However all is not well if you go to Section 9 of the IB data booklet.
The IB data booklet shows that the ionic radius of Fe2+ (61 x 10−12 m) is indeed smaller than the ionic radius of Mn2+ (83 x 10−12 m) but the ionic radius of Co2+ (65 x 10−12 m) is actually greater (not smaller) than the ionic radius of Fe2+. This means that the charge density of Co2+ should be less than the charge density of Fe2+ and the hydration energy would be predicted to be less exothermic for Co2+ compared to Fe2+ which is not the case.
The problem with the argument given above lies in the statement that as the number of protons increases the radius decreases as you move across the period as the outer electrons are in the same energy level. This is true for the atomic radius of the elements in the periods Li - F and Na – Cl but only just holds true for the transition metals.

Atomic radii for elements in Periods 2 and 3. Note that even if the metallic radii are taken for the metals (as in the 2009 IB data booklet) the trend is still the same.

Atomic radii for the first row transition elements
In fact the transition metal atoms have rather similar atomic radii which only gradually decrease across the period (although copper atoms have a greater atomic radius than nickel atoms). This is one of the reasons why transition metals readily form alloys. As for the 2+ ions the ionic radius does not really follow a pattern at all. When studying periodicity you do look at the size of ions but only tend to compare isoelectronic ions such as Na+, Mg2+ and Al3+ which do decrease in size, but you do not generally compare the size of similarly charged ions such as Na+, Mg+ and Al+. The values for the radii of the 2+ first row transition metal ions which are given in the IB data booklet are as follows:
.png)
Ionic radii of some dipositive first row transition metal ions
I would suggest that you cannot really answer this question based on your knowledge of IB chemistry without the data booklet as there is no way you could correctly predict which of any two given dipositive transition metal ions will have the smallest radius. Even using the values given for the ionic radii in the IB data booklet might still lead to an incorrect prediction since Co2+ has a larger ionic radius than Fe2+ and yet Co2+ still has a greater exothermic enthalpy of hydration than Fe2+. So the relationship between the predicted relative charge densities and hydration energy is also not clear cut, assuming the ionic radii given in the data booklet are correct. In fact the ionic radius of the dipositive transition metal ion in a complex as opposed to the free ion in the gaseous state is not a fixed value as inferred from the IB data booklet but varies according to the coordination number of the compound, the shape of the compound and on whether it is a high-spin or low-spin complex (see 13.1 First-row d-block elements), i.e. on how many unpaired electrons it contains. If there is a decrease in ionic radii in the gaseous ions as the atomic number increases then one would expect there to be a similar increase in the quantitative value of the hydration enthalpies. From the table below it can be seen this is generally true but not completely true.
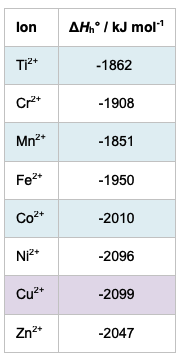
Enthalpies of hydration of some d-block dipositive ions (values from wiredchemist)

 IB Docs (2) Team
IB Docs (2) Team