4.1 Ionic bonding & structure
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 4.1 Ionic bonding & structure. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.

 Learning outcomes
Learning outcomes
After studying this topic you should be able to:
 Understand:
Understand:
- Cations (positive ions) are formed by metals losing valence electrons.
- Anions (negative ions) are formed by non-metals gaining electrons.
- The electron configuration of the atom determines the number of electrons lost or gained.
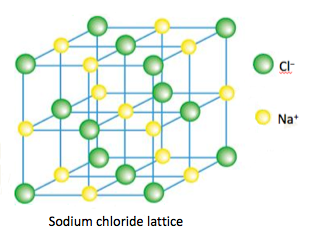
- Ionic bonds are due to electrostatic attractions between oppositely charged ions and are equal to the sum of all the attractive and repulsive forces present between ions in the lattice.
Under normal conditions, ionic compounds are usually solids with lattice structures.
Apply your knowledge to:
- Deduce the formula and name of an ionic compound from its component ions, including polyatomic ions.
(Note that you should be familiar with with the formulas and the names of the following polyatomic ions: NH4+, OH–, NO3–, HCO3–, CO32–, SO42– and PO43–.) Explain the physical properties (volatility, electrical conductivity and solubility) of ionic compounds in terms of their structure.
Relationships & vocabulary
Nature of Science
Natural phenomena can be explained by using theories. For example, molten ionic compounds conduct electricity but solid ionic compounds do not - this can be explained in terms of the breaking of ionic lattices.
International-mindedness
For examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 4 & 14: Chemical bonding & structure.
Vocabulary
| electrovalent | cation | anion | ionic bond |
| electron affinity | lattice enthalpy | polyatomic ion |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
 Something that always amazes me is how, having literally just covered Topic 3 on Periodicity, some teachers and many books seem to forget all that they have taught on that topic when they introduce ionic bonding. Almost all books and I suspect many teachers make the most elementary mistakes which you should recognise from the material covered under periodicity as being completely wrong.
Something that always amazes me is how, having literally just covered Topic 3 on Periodicity, some teachers and many books seem to forget all that they have taught on that topic when they introduce ionic bonding. Almost all books and I suspect many teachers make the most elementary mistakes which you should recognise from the material covered under periodicity as being completely wrong.
These mistakes include:
![]() Showing how sodium atoms give an electron to a chlorine atom when the reaction is actually between sodium metal and gaseous chlorine molecules, Cl2 (see the image on the right showing sodium metal burning in chlorine gas).
Showing how sodium atoms give an electron to a chlorine atom when the reaction is actually between sodium metal and gaseous chlorine molecules, Cl2 (see the image on the right showing sodium metal burning in chlorine gas).
Na(s) + ½Cl2(g) → NaCl(s)
![]() Making statements such as sodium atoms "want to lose an electron to gain a noble gas configuration (or arrangement)". Apart from the fact that sodium atoms do not have wants, much more important is that they do not easily lose an electron. The process is endothermic to the tune of + 496 kJ mol-1.
Making statements such as sodium atoms "want to lose an electron to gain a noble gas configuration (or arrangement)". Apart from the fact that sodium atoms do not have wants, much more important is that they do not easily lose an electron. The process is endothermic to the tune of + 496 kJ mol-1.
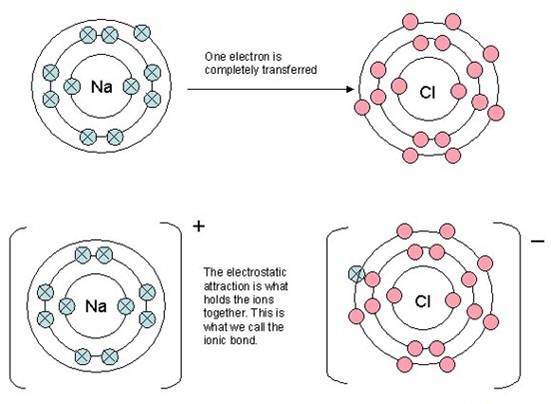
A typical way of showing ionic bonding that can be found in many books
![]() Showing atoms of sodium and chlorine to both be the same size, i.e. with identical atomic radii.
Showing atoms of sodium and chlorine to both be the same size, i.e. with identical atomic radii.
![]() Showing a chloride ion the same size as a chlorine atom, and in some cases the same size as a sodium ion.
Showing a chloride ion the same size as a chlorine atom, and in some cases the same size as a sodium ion.
![]() Showing all the energy levels in both the sodium and chlorine atoms to be equally spaced.
Showing all the energy levels in both the sodium and chlorine atoms to be equally spaced.
![]() Showing the electrons separately in the first energy level and in pairs in the subsequent energy levels.
Showing the electrons separately in the first energy level and in pairs in the subsequent energy levels.
![]() Ignoring the changes of state that take place during the reaction.
Ignoring the changes of state that take place during the reaction.
So before you answer questions on ionic bonding – just reflect a while!
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Ionic bonding & structure.
For short-answer questions see Ionic bonding & structure questions.
For a data response question on ionic bonding see Data response question with the worked answers on a separate page Data response answers.
Other resources
1. A very simple UK A Level revision video which shows the electron transfers taking place between neutral atoms to form ions. It leads on to a second video which discusses giant ionic lattices and how these explain the properties of ionic compounds. Remember that "dot and cross" diagrams are the UK way of describing Lewis structures!
2. A video by the Khan academy on bonding which is supposed to be educational. To me this exemplifies all that is wrong about how ionic bonding is sometimes taught. No questions are asked of students and the facts given are often simply wrong.
![]() Iconic (!) bonding (Khan academy)
Iconic (!) bonding (Khan academy)

 IB Docs (2) Team
IB Docs (2) Team 














