IE graph(s)
Explaining the shape of ionization energy graphs
Explaining the shapes of ionization energy graphs tends to be more of a problem at Standard Level than Higher Level. This is mainly because the best explanation involves sub-levels and although ionization energies provide evidence for sub-levels the trends are only really mentioned in the AHL sub-topic 12.1: Electrons in atoms. Even so, Higher Level students also often show some misunderstanding of the basic principles.
It can be instructive to plot your own graph of ionization energy against atomic number for the 1st ionization energies of the first twenty elements.
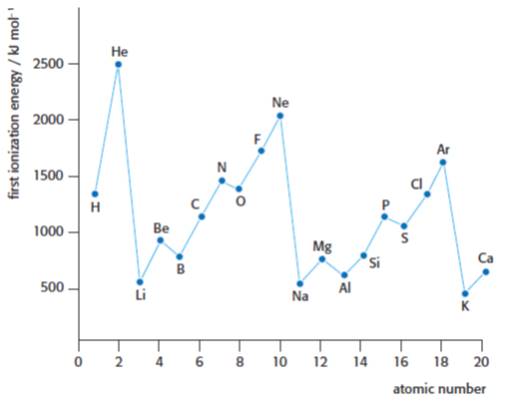
This helps you to fix the pattern in your own mind and also gives you some idea of which elements have high values, which have low values and the general trends etc.
It is then worth looking at a graph of the first ionization energy plotted for all the elements (well, just the first 95!).
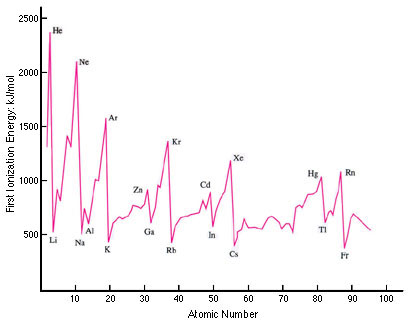
From this graph you can see that the pattern continues repeating (including the transition metals which do not show up in the first 20 elements) which clearly illustrates the term ‘periodicity’1. If you look at a graph of the second ionization energies against atomic number and compare it with the first ionization energy graph it shows that the pattern is very similar but shifted by one atomic number so that, for example, Li+(g) now replaces helium for the highest value and the graph drops rapidly for Be+(g). Higher Level students also need to be familiar with the graph (using a logarithmic scale this time) for all successive 19 ionization energies of potassium.
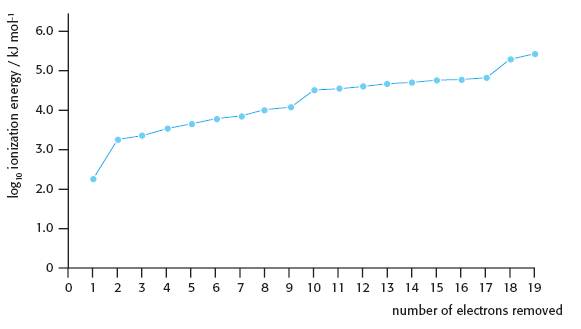
In all cases the 2.8 pattern etc. is obvious. So why then do many students then have problems explaining it? (Their weakness in understanding usually shows up in the longer-answer responses required in Paper 2.)
Footnote
1 It is interesting, and very strange, that although sub-topic 3.2 mentions "horizontal and vertical trends" it does not use the word periodicity. Make sure you understand that periodicity refers to a repeating pattern of physical and/or chemical properties as the atomic number increases.
Common problems
Some of the common problems that students seem to have with ionization energies include:
1. Not understanding the essential definition that ionization energies refer to the gaseous state and refer to one mole of atoms so that the units are in kJ mol-1.
i.e. M(g) → M+(g) + e−
2. Not understanding that ionization energies are an endothermic process. Sodium atoms do not ‘want’ to lose an electron to gain a noble gas configuration. A considerable amount of energy (approximately 500 kJ mol-1) has to be put in to remove the outer electron.
3. Not understanding that the electronic configurations actually relate to energy levels (rather than the often used analogy of planets orbiting the sun). Lithium has a much lower first ionization energy that helium because the outer electron is in a much higher energy level so much less energy is required to remove it. Because the difference in energy is so great the gap between the 1st and the 2nd level is much greater than the gap between the 2nd and 3rd levels and the 3rd and 4th levels etc. This is often not emphasized in text books or on websites (e.g. Revisionworld, see diagram below) where, for example, when they draw the electronic arrangement for sodium they wrongly show equal gaps between the levels.

4. Not understanding that pairing the second electron up within an orbital actually causes some repulsion. This concept gets hidden because a ‘full shell’ has the highest value (e.g. He is much higher than H). The reason He is much higher than H is not because the shell is full as such but because helium has an extra proton in its nucleus which make the attraction between the nucleus and the outer shell much stronger. Of course the lithium nucleus, which contains three protons, would exert an even stronger attraction to the three electrons if they were all in the same energy level but now the outer electron is in a much higher energy level so easier to remove.
5. Many students find difficulty in explaining why there are dips between Be(g) and B(g) and between N(g) and O(g) in the graph as this goes against the general trend of increasing ionization energies across a period. The reason why the trend is generally increasing is that as extra electrons are added to the outer energy level so too are extra protons being added to the nucleus. This increases the attraction of the nucleus to the outer energy level as the atomic number increases across a period and so makes it harder to remove an electron. The dips provide evidence for sub-shells. Both SL and HL students need to know about sub-levels to deduce electron configurations but only HL students need to be able to explain the dips in the graph.
6. Higher Level students often give two major erroneous explanations in their answers which show considerable misunderstanding.
The first occurs when students are asked to explain why boron has a lower first ionization energy level than beryllium. They often state that beryllium has a full sub-level so this confers some stability. The correct answer is that boron has the configuration 1s22s22p1. The 2p electron is in a sub-level with a higher energy than the 2s electrons so less energy is required to remove it.
The second error occurs when they try to explain why oxygen has a lower first ionization energy than nitrogen. Again they often talk about the stability of a half-filled sub-level. To explain it correctly first give the electronic configuration and this time also distinguish between the three p orbitals. So the electronic configuration of nitrogen is 1s22s22px12py12pz1 and the electronic configuration of oxygen is 1s22s22px22py12pz1. If you understand that adding an extra electron to an orbital causes repulsion then you can quickly see that it will require less energy to remove the second electron from the 2px orbital in the case of oxygen compared to nitrogen, where there are no longer two electrons repelling each other within the same orbital in the same sub-level.

 IB Docs (2) Team
IB Docs (2) Team