3.1 The periodic table
Written specifically for students this page covers all the syllabus content of Topic 3.1. It encourages you to think critically and provides many questions with full answers to test your knowledge & understanding.

 Learning outcomes
Learning outcomes
 After studying this topic you should be able to:
After studying this topic you should be able to:
Understand:
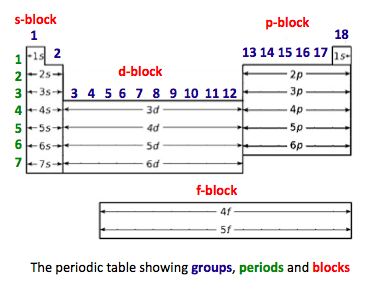
- The periodic table is arranged into four blocks. These are associated with the four sub-levels - s, p, d, and f.
- The periodic table consists of groups arranged in vertical columns and periods arranged in horizontal rows.
- The number of the period (n) is the outer energy level that is occupied by electrons.
- The number of the principal energy level (n) and the number of the valence electrons in an atom can be deduced from its position on the periodic table.
- The positions of metals, non-metals and metalloids are shown in the periodic table.
Apply your knowledge to:
- Deduce the electron configuration of an atom from the element’s position in the periodic table, and vice versa.
Relationships & vocabulary
Nature of science
Evidence for scientific theories is obtained by making and testing predictions.
The periodic table is an example of how subjects are organised by scientists based on subject and function. Early models of the periodic table enabled Mendeleev (and later Moseley) to predict the existence and properties of elements that had not yet been discovered.
International-mindedness
The development of the periodic table involved scientists from different countries building upon the foundations of each other’s work and ideas and took many years.
For examples and more links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 3 & 13: Periodicity.
Vocabulary
| group | period | periodicity |
| transition element (metal) | lanthanoid | actinoid |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
 The wide variety of periodic tables available has been referred to already in the introduction to Topics 3 & 13 : Periodicity. Periodic tables also provide a good example of International-Mindedness in Chemistry as they appear in many different languages and yet are instantly understandable by a chemist. One point that may be worth reflecting upon is that periodic tables are called something slightly different in Russian. Russian speakers refer to the periodic table as the Mendeleev table (see left). We keep the names of Western scientists in many of our discoveries. For example, Faraday's Laws, Avogadro's constant and Le Chatelier's Principle. Why is it that we omit Mendeleev from the name of 'his' table when it is one of the most important concepts in the whole of Chemistry?
The wide variety of periodic tables available has been referred to already in the introduction to Topics 3 & 13 : Periodicity. Periodic tables also provide a good example of International-Mindedness in Chemistry as they appear in many different languages and yet are instantly understandable by a chemist. One point that may be worth reflecting upon is that periodic tables are called something slightly different in Russian. Russian speakers refer to the periodic table as the Mendeleev table (see left). We keep the names of Western scientists in many of our discoveries. For example, Faraday's Laws, Avogadro's constant and Le Chatelier's Principle. Why is it that we omit Mendeleev from the name of 'his' table when it is one of the most important concepts in the whole of Chemistry?
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Periodic table.
For short-answer questions see Periodic table questions.
More resources
1. A rather simplistic short history of Mendeleev's discovery of the periodic table from the Science Channel videos.
2. A better video on the same topic from the BBC's Chemistry - a volatile history.
3. Martin Poliakoff shows you some of his collection of periodic tables and discusses why periodic tables are useful. Basically it is an introduction to the University of Nottingham series of videos about the periodic table.
4. An absolute classic by Tom Lehrer. If you want to learn the names of the elements (at least those that were known in 1967) just set them to music.
\
![]() The elements song - Tom Lehrer
The elements song - Tom Lehrer

 IB Docs (2) Team
IB Docs (2) Team 










