Condensation polymers
 A.9 Condensation polymers (2 hours)
A.9 Condensation polymers (2 hours)
Pause for thought
It is worth looking at Kevlar in some detail, as one of the statements about Kevlar in the syllabus is a little confusing. Kevlar has been known since 1965. It was discovered by Stephanie Kwolek (1923 - 2014), an American chemist of Polish origins, whilst she was working for DuPont. Kevlar (polyparaphenylene terephthalamide) is a polyamide made from condensing 1,4-diaminobenzene with benzene-1,4-dicarbonyl chloride.
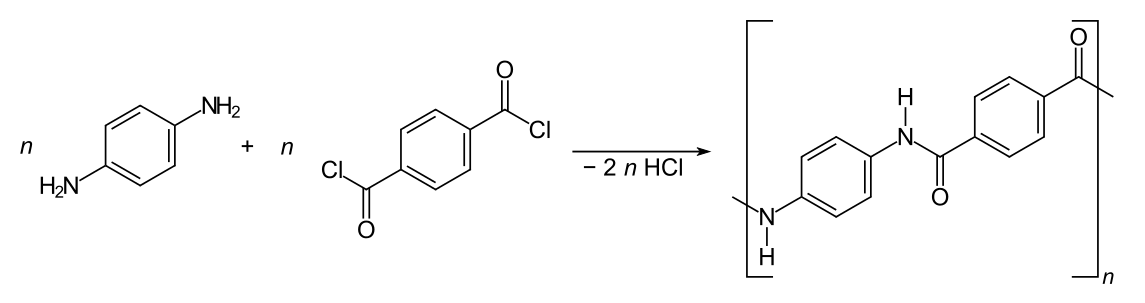
The formation of Kevlar from 1,4-diaminobenzene and 1,4-dicarbonyl chloride
It is actually quite expensive to make industrially as it needs to be kept in a solution of concentrated sulfuric acid during both its synthesis and when its fibres are being spun. This is to help prevent it forming into solid layers through cross-linking hydrogen bonds. Once the sulfuric acid is removed the three-dimensional cross-linked layers are then formed and it is this that gives Kevlar its immense strength. It is said to be more than five times stronger than steel although materials made of graphene are now even stronger. A recent paper reported that spiders sprayed with graphene nanoparticles produce a silk fibre, which is the strongest fibre ever measured.
One of the problems of polyamides like Kevlar is that they can be hydrolysed back to their monomers by acid solution. For this reason it is not a good idea to let car battery acid come into contact with materials made of nylon (such as climbing ropes). Kevlar is in fact more resistant to hydrolysis than nylon but concentrated sulfuric acid will break down the hydrogen bonding cross-links in Kevlar and severely weaken the structure. The syllabus states” The hydrogen bonds between O and N can be broken with the use of concentrated sulfuric acid”. This of course is not strictly true. The hydrogen bonds are not between the oxygen and nitrogen atoms but between the oxygen atoms of peptide linkages and the hydrogen atoms covalently bonded to the nitrogen atoms belonging to adjacent peptide linkages.

The structure of Kevlar (one repeating unit is shown in bold)
Kevlar has many uses. It is used to make the bulletproof vests employed by the military and police forces. Protective clothing such as motorcycle gear includes Kevlar to prevent cuts and abrasions in motorcycle accidents. It is also used to reinforce composite materials, in various musical instruments and to make ropes. There is good page of information on Kevlar produced by Bristol University in their series Molecule of the Month. Note that Kevlar can also act as a lyotropic liquid crystal (see A.4 Liquid crystals.)
Nature of Science
Will future civilisations classify our current era as the 'Polymer Age' as a way of continuing to describe how humans use science to manipulate matter as has been done with the Stone Age, Iron Age and Bronze Age in the past?
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesInclude green chemistry considerations of forming and using polymers. International-mindednessWhat are the roles of science, economics and politics in the research and development of new polymers? |
Teaching tipsThis is a very straightforward sub-topic to teach. Students need to be aware of the difference between an addition polymer and a condensation polymer and realise that to form a condensation polymer both monomers must contain at least two reactive functional groups. The classic two cases are the polymerisation of dicarboxylic acids with diols to form polyesters and the polymerisation of diamines with dicarboxylic acids or diacyl chlorides to form polyamides. If students understand which bonds are broken to form the polyester and polyamide links then they should be able to draw the repeating unit of the polymer. They should also be able to deduce the balanced equation for the reaction when n molecules of one monomer are reacted with n molecules of another molecule and realise that the balanced equation will contain (2n-1) molecules of the inorganic product which is usually water or hydrogen chloride. Use poly(ethyl benzene-1,4-dicarboxylate) (also known as polyethylene teraphthalate, PET, PETE, Terylene or Dacron) as an example of a polyester and it can be fun for students to make their own nylon 6,6 as an example of a polyamide. You will need to discuss the structure and cross-linking that can occur in Kevlar due to the hydrogen bonding between the oxygen of one peptide bond with a hydrogen atom attached to the nitrogen atom of another peptide bond. This results in a very ordered and strong three-dimensional structure. In strong acid solution the oxygen and nitrogen atoms in the amide linkage become protonated. This means they can no longer form hydrogen bonds so it leads to the breakdown of the cross-linkage and so weakens the strength of the Kevlar (see 'Pause for thought' above). Finally relate the formation of condensation polymers to green chemistry by calculating the atom economy (see Environmental impact - plastics) | Study GuidePage 120 QuestionsFor ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Condensation polymers. For short-answer questions see Condensation polymers questions together with the worked answers on a separate page Condensation polymers answers. Vocabulary listcondensation polymerisation Practical work |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts
Other resources
1. Jared Haymann from Elon University shows how nylon can be made in the lab.
2. A simple but clear video on condensation polymerization by Fuseschool.
3. A demonstration by DuPont on how gloves made from Kevlar are much more resistant to cuts than gloves made from cotton or leather.
![]() Demonstration of the strength of Kevlar
Demonstration of the strength of Kevlar

 IB Docs (2) Team
IB Docs (2) Team 













