Solar energy answers

 Answers to questions on Solar energy
Answers to questions on Solar energy
Answers to Solar energy questions
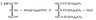
(b) Because of their high molar masses they are too involatile for use as diesel fuel.

(d) It acts as a catalyst (i.e. it provides a reaction pathway with a lower activation energy).
(e) To increase the yield of transester by shifting the position of equilibrium towards the product side.
2. (a) C6H12O6 → 2C2H5OH + 2CO2
(b) The enzyme in the yeast (zymase) responsible for the fermentation is destroyed when the alcohol concentration exceeds about 15%. Fractional distillation can be used to obtain 96% pure alcohol. (To obtain 100% pure ethanol the 4% of water needs to be removed with a drying agent, such as anhydrous magnesium sulfate).
3. (a) 1 kg of ethanol contains 1000 / 46.1 mol
Specific energy = (1000 / 46.1) x 1367 = 2.97 x 104 kJ kg-1
1 kg of octane contains 1000 / 114.26 mol
Specific energy = (1000 / 114.26) x 5470 = 4.79 x 104 kJ kg-1
1 kg of octane produces 1.6 times as much energy as 1 kg of ethanol when completel combusted.
(b) Ethanol is not a fossil fuel so is replaceable.
Carbon dioxide is absorbed by plants so when ethanol is burned there is no net increase of carbon dioxide gas in the atmosphere, so it does not contribute to global warming.
4. (a) The extensive conjugation (alternate double and single bonds).
(b) Chlorophyll absorbs light in both the low (red) and high (blue) regions of the visible spectrum so transmits green light, which lies in the middle of the visible spectrum.
Download the Answers to Solar energy questions

 IB Docs (2) Team
IB Docs (2) Team