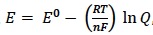Electrochemistry, rechargeable batteries & fuel cells
 C.6 Electrochemistry, rechargeable batteries & fuel cells
C.6 Electrochemistry, rechargeable batteries & fuel cells
(4 hours)
Pause for thought
The first crude fuel cells were actually invented as long ago as 1838 by William Grove, a Welshman and independently by Christian Schönbein from Germany.
It was the NASA space programme that really developed them to become a viable technology. The space shuttle contains three fuel cells, which operate as independent power sources. These use oxygen and hydrogen gas and an electrolyte of potassium hydroxide. Each one is capable of supplying 12 kW peak and 7 kW maximum continuous power supply which is ample for the average power consumption of the shuttle. As well as providing electrical power and heat, the product, water, is used by the astronauts for drinking.
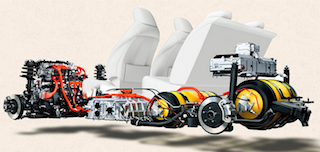
Cut-away of the Toyota Mirai showing the fuel cell stacks beneath the driver and passenger seats and the yellow hydrogen fuel tanks in the rear.
Only now are fuel cells being seriously used to power cars. This year Toyota plans to build 700 cars running on hydrogen fuel cells. The car, the Toyota Mirai, has a range of 312 miles (502 km) from a full tank. The first cars went on sale in the US in August 2015 at a basic cost (before any government subsidies) of $57 500. The relatively high cost and the current lack of hydrogen fuel stations are two initial hurdles that will need to be overcome. Ultimately vehicles powered by fuel cells (FCVs) may well prove to be the best answer to providing transport for the masses that does not rely on fossil fuels and so significantly reduce the emission of polluting greenhouse gases.
Nature of science
Redox reactions can be used as a good source of electricity but the disposal of batteries can cause environmental problems.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesConsider a battery to be a portable electrochemical source comprising of one or more voltaic (galvanic) cells connected in series. The Nernst equation is given in Section 1 of the data booklet. Consider hydrogen and methanol as fuels for fuel cells and consider the operation of fuel cells under both acid and alkaline conditions. Familiarity with proton-exchange membrane, PEM, fuel cells is expected. Use the Geobacter species of bacteria as an example that can be used in some cells to oxidize ethanoate ions, CH3COO−, under anaerobic conditions. Consider the lead–acid storage battery, the nickel–cadmium, NiCd or NiCad, battery and the lithium–ion battery. Familiarity with the half-equations at the anode and cathode and the uses of the different cells is expected. International-mindednessThe ways in which spent batteries are disposed of and/or recycled vary from country to country. |
Teaching tipsThis sub-topic follows on neatly from Topics 9 & 19 from the core/AHL. Essentially it is the practical application of voltaic cells so does require some memory work as well as a deeper understanding. The key to the sub-topic is either knowing or being able to deduce the equations for the half-reactions taking place in the different types of rechargeable batteries and fuel cells. I start by revising how voltaic cells work and how to calculate the electromotive force for the whole cell (EMF or E⦵total) from the standard electrode potentials of the two half-cells. Then go into the specific details for the lead-acid, Ni/Cd and Li-ion rechargeable batteries. Students should realise that when batteries recharge the reaction occurring is the reverse of when it discharges. Fuel cells are expensive but have considerable potential. Start with the hydrogen fuel cell and stress that the same overall reaction occurs irrespective of whether the electrolyte is acidic or alkaline but that the half-reactions are different. When methanol is used as the fuel carbon dioxide and water are the products of oxidation. However if the oxidation is carried out anaerobically, as in a microbial fuel cell, then carbon dioxide, hydrogen ions and electrons are formed instead. By using a membrane to allow the passage of the hydrogen ions and the external circuit to allow the flow of electrons a microbial fuel cell can be used to provide a sustainable energy source using waste water and bacteria. Explain the thermodynamic efficiency of a fuel cell including why, in the hydrogen fuel cell, ΔG⦵ is smaller in quantitative value than ΔH⦵ as work needs to be done to overcome the negative entropy change when gaseous hydrogen and oxygen turn into liquid water. One way to ensure they fully understand this is to get them to work out the value for ΔS⦵ (see 2(d) in the questions). The syllabus states that the Nernst equation can be used to calculate the potential of a half-cell in an electrochemical cell, under non-standard conditions. This is not strictly true. It can be used to calculate the potential for non-standard concentrations but not when just the temperature is changed as then the expression – (RT / nF) ln Q = 0. Give your students plenty of practice at working out the EMF for cells with different concentrations including concentration cells (where only the concentration of the ions in the solutions of otherwise identical half-cells changes). | Study GuidePage 163 QuestionsFor ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Electrochemistry, rechargeable batteries & fuel cells. For short-answer questions see Electrochemistry, rechargeable batteries & fuel cells questions together with the worked answers on a separate page Electrochemistry, rechargeable batteries & fuel cells answer . Vocabulary listNernst equation Practical work
|
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A good description by BASF on how lithium-ion batteries work.
![]() How lithium-ion batteries work
How lithium-ion batteries work
2. Another good description - this time on how a lead-acid battery works by Bill Hammack from the University of Illinois.
3. An animated overview of fuel cells by the Naked Science Scrapbook which provides a good introduction to the different types of fuel cells and why they are useful (but does not include the half-equations).
4. This American Chemistry Society video shows how waste water can be used to produce electricity using microbial fuels cells.
![]() Using microbial fuel cells to generate electricity
Using microbial fuel cells to generate electricity

 IB Docs (2) Team
IB Docs (2) Team