pH regulation of the stomach
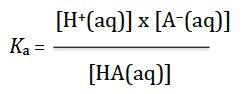

 D.4 pH regulation of the stomach (3 hours)
D.4 pH regulation of the stomach (3 hours)
Pause for thought
Parts of this sub-topic could be a real problem if you are a Standard Level student - somehow there seems to be a mismatch between the content in the core topics and the content of the core part of the option. In Topic 8: Acids and bases, which forms part of the main core, you do cover the definition of pH, but you do not cover acid or base ionization constants, Ka or Kb, or pKa or pKb, or the relationship between pKa and pKb. Neither do you learn anything about buffer solutions, nor pH curves so you will are not familiar with the half-equivalence point. In sub-topic 18.3, even Higher Level students are not required to solve buffer calculations and yet in this core sub-topic, Standard Level students as well as Higher Level students are expected to solve problems involving buffer solutions by using the Henderson-Hasselbalch equation.
Because you will always have access to the data booklet in the examination for this option you do not need to remember the Henderson-Hasselbalch equation. However to expect you to apply this equation without any understanding seems contrary to the good teaching the IB expects. In fact, in the past I have never used the equation as such as I prefer students to always work from first principles rather than learn or use an unnecessary equation. All you need to know to solve buffer calculations is that pH = −log10 [H+(aq)] (Topic 8) and from this you can deduce that pKa = −log10 Ka. You also need to know that for a weak acid in water HA(aq), the acid dissociation constant, Ka can be expressed just like any other equilibrium constant (Topic 6), i.e.
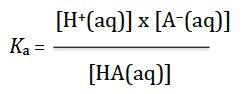
Although not necessary to solve problems, the Henderson-Hasselbalch equation can easily be derived from this.
If logarithms to the base ten are then taken the equation becomes

Subtract log10 Ka and log10 [H+(aq)] from both sides so it becomes
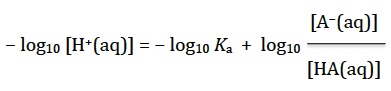
Substituting the definitions for pH and pKa gives the normal expression for the Henderson-Hasselbalch equation

I suspect (hope?) that for Standard Level students the calculations will mainly focus on the special case when the concentration of the salt is equal to the concentration of the acid, i.e. at the half-equivalence point when a weak acid is titrated with a strong base. At this point [A−(aq)]/[HA(aq)] = 1, so log10 ([A−(aq)]/[HA(aq)]) = zero and the pH = pKa.
In fact you should realise that there is no need to use the Henderson-Hasselbalch equation at all to arrive at this conclusion as it comes directly from the equilibrium expression for the acid dissociation. When [A−(aq)] = [HA(aq)] then Ka is simply equal to [H+(aq)] and hence pH = pKa.
Nature of Science
The symptoms of dyspepsia include the overproduction of stomach acid. There are several different ways in which this can be treated. These includes the prescription of antacids to neutralize the acid, the use of H2-receptor antagonists and the use of proton pump inhibitors which work by preventing the production of stomach acid. The effectiveness of these different treatments can be assessed through collecting data by sampling and trialling.
Learning outcomes
After studying this topic students should be able to:
Understand
- Antacids reduce excess stomach acid.
- The active form(s) of a drug after it has been processed by the body are called active metabolites.
Apply their knowledge to:
- Explain how excess acidity in the stomach can be reduced by the use of different bases.
- Construct and balance equations for neutralization reactions and apply theses equations using their stoichiometry.
- Solve buffer problems using the Henderson–Hasselbalch equation.
- Explain how compounds, such as ranitidine (Zantac), can be used to inhibit stomach acid production.
- Explain how compounds, such as omeprazole (Prilosec) and esomeprazole (Nexium), are used to suppress acid secretion in the stomach.
Clarification notes
Examples of antacid compounds include calcium hydroxide, magnesium hydroxide, aluminium hydroxide, sodium carbonate and sodium bicarbonate.
The structures for ranitidine and omeprazole can be found in Section 37 of the data booklet.
Note that the current guide (dated February 2015) says that the structure of esomeprazole (not omeprazole) is given. In fact in Section 37 of the current data booklet (Version 2, dated June 2014) only the structure of omeprazole is given, not esomeprazole. Omeprazole is actually a racemic mixture, whereas esomeprazole is a specific enantiomer, but enantiomers are not on the core programme.
International-mindedness
The need for pH regulation of the stomach can be affected by the diet, lifestyle and genetics etc. of different cultures.
Teaching tips
This sub-topic focuses on three different ways to combat excess acidity in the stomach (dyspepsia). Students need to know about the normal pH of gastric juices, which lies in the region of about 1-2.5. The first way has basically been covered already in Topic 8: Acids and bases as it involves neutralising the excess acid with a base. Get students to write the equations for the reactions of the oxides and hydroxides of magnesium and aluminium with hydrochloric acid and also the reactions of carbonates and hydrogencarbonates with hydrochloric acid. They will also need practice with questions to calculate which particular antacids will be able to neutralise the most acid.
Most commercial antacids also include other ingredients. The previous programme included the role of the neutralising layer of alginates to prevent stomach acid from rising up the oesophagus to cause heartburn and the role of anti-foaming agents such as polydimethylsiloxane (PDMS), known as dimethicone, which help to reduce trapped gases (wind). It would appear that knowledge of alginates and anti-foaming agents and is no longer required.
The second method involves the use of drugs like ranitidine. These bind to the H2 histamine receptor in the cells of the gastric lining and prevent secretion of the acid in the stomach. The third method involves the use of drugs (e.g. omeprazole and esomeprazole), which restrict the amount of acid produced by inhibiting a particular enzyme, known as the gastric proton pump, involved in its production. Note that since the syllabus was produced both PPIs and H2 receptor blockers have been shown to have potentially serious side effects - see The danger of prolonged use of proton pump inhibitors and Sales of Zantac® suspended.
Finally you will need to teach about buffer solutions and give examples of buffer calculations. For Standard Level students this will require a considerable amount of new background theory - see 'Pause for thought'
Study guide

Pages 158 & 159
Questions
For ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: pH regulation of the stomach.
For short-answer questions see pH regulation of the stomach questions together with the worked answers on a separate page pH regulation of the stomach answers.
Vocabulary list
dyspepsia
antacid
metabolite
Henderson-Hasselbalch equation
ranitidine
omeprazole
esomeprazole
H2 receptor antagonist
proton pump inhibitor
Teaching slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Other resources
1. A helpful article from Patient UK which explains what antacids are and how they work.
2. A rather nice explanation, including an animation, of how ranitidine works by binding to the H2-histamine receptor.
3. Another animation to show how proton pump inhibitors work.
![]() Action of proton pump inhibitors
Action of proton pump inhibitors

 IB Docs (2) Team
IB Docs (2) Team 






















