Preparation of nylon 6,6
 Introduction
Introduction
Nylon 6,6 was the first type of nylon to be made. It was produced by Wallace Carothers (1896 – 1937) in 1935 when he was working for Dupont at Wilmington, Delaware. Carothers suffered from depression and despite the obvious commercial success of nylon he took his own life by cyanide poisoning in 1937.
Nylon (the name comes from combining New York and London) is the generic name for many polyamides. It has a multitude of uses including ‘nylons’ which were introduced as women’s stocking in 1940. Due to a shortage of silk in World War II nylon was used to make parachutes. Other applications include climbing ropes, machine parts, packaging, clothing and fishing lines.

A nylon rope used for mountaineering
Teacher's notes
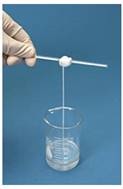 This practical provides a simple but fun example of how two monomers, both with two reactive functional groups, can condense together to form a condensation polymer. It does not take long to do but will help students to visualise condensation polymerisation reactions and also give them practice at writing the relevant equations. You can challenge your students to see who can make the longest rope without breaking it.
This practical provides a simple but fun example of how two monomers, both with two reactive functional groups, can condense together to form a condensation polymer. It does not take long to do but will help students to visualise condensation polymerisation reactions and also give them practice at writing the relevant equations. You can challenge your students to see who can make the longest rope without breaking it.
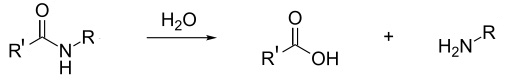
In the presence of strong acids hydrolysis occurs. The peptide linkage in nylon is broken and the polymer reverts back to the carboxylic acid and amine monomers. This also happens with Kevlar even though the main reaction listed on the syllabus ( A.9 Condensation polymers) between Kevlar and concentrated acids is the destruction of the hydrogen bonding responsible for its three-dimensional structure.
 Student worksheet
Student worksheet
PREPARATION OF NYLON 6,6
The aim of this experiment is to demonstrate how a polyamide can be prepared by taking two monomers each of which contains two reactive functional groups. The particular polymer formed is nylon 6,6 – a polyamide. It is formed from reacting hexanedioyl chloride (adipoyl chloride) ClOC−(CH2)4−COCl with 1,6-diaminohexane, H2N−(CH2)6−NH2. The acid chloride groups condense with the amine groups to release hydrogen chloride and form the repeating unit of nylon 6,6.

repeating unit of nylon 6,6
Because two immiscible solutions of the two monomers are used the reaction takes place at the interface of the two solutions provided they do not become mixed.
ENVIRONMENTAL CARE:
The organic chemicals used and the product should be placed in the organic waste after the experiment is completed.
SAFETY:
The solutions used are corrosive and toxic. Avoid breathing the fumes. The reaction creates fumes of hydrogen chloride and hydrochloric acid as a by-product. Use protective gloves and goggles and avoid touching the strands of nylon 6,6 formed with your ungloved hands.
PROCEDURE:
Place 3 cm3 of a solution of 0.5 mol dm-3 1,6-diaminohexane in 0.5 mol dm-3 sodium hydroxide in a 50 cm3 beaker. Holding the beaker on a slant carefully add 3 cm3 of a 0.25 mol dm-3 solution of hexanedioyl chloride in hexane to it. The two solutions should not be allowed to mix. Using a glass rod with a hook on the end withdraw the film that forms at the boundary and carefully continue to wind the film as a strand around the glass rod. When you have withdrawn a sufficient length wash the filament thoroughly in distilled water then unwind it using gloves to obtain a long length of nylon 6,6.
QUESTIONS:
1. Explain why the two layers do not mix unless they are shaken.
2. Write the balanced equation for the reaction of n molecules of hexanedioyl chloride with n molecules of 1,6-diaminohexane to form nylon 6,6.
3. Nylon 6,10 can be made by reacting decanedioic acid (sebacic acid), HOOC−(CH2)8−COOH with 1,6-diaminohexane. Draw the repeating unit of nylon 6,10 and write a balanced equation for the reaction.
This worksheet can also be downloaded from:

 IB Docs (2) Team
IB Docs (2) Team