Redox reactions of vanadium
 Introduction
Introduction
This experiment addresses two different areas of the Additional Higher Level (AHL) material. Topic 13.1 First-row d-block elements is concerned with the chemistry of the first-row transition elements and topic 19.1 Electrochemical cells (AHL) covers standard electrode potentials. The electronic configuration of vanadium is clearly on the syllabus but although students are expected to be able to work out the oxidation state of vanadium in compounds such as NH4VO3, VSO4 and V2O5 they do not need to learn any of the specific chemistry of the compounds of vanadium.
This experiment uses the characteristic colours of compounds of vanadium with different oxidation states to illustrate redox chemistry and also it encourages students to use standard electrode potentials to predict which species will be oxidised and which reduced when they are reacted together. It is a good experiment to increase students' understanding of theory and also very enjoyable due to the obvious colour changes. From a safety point of view this is a very simple experiment with the only real hazard being the use of concentrated sulfuric acid. Make sure that all the waste is transferred to the heavy metals waste container.
There is a good short video produced by the Open University which illustrates well the colours of the different oxidation states of vanadium.
 Student worksheet
Student worksheet
REDOX REACTIONS OF VANADIUM
This practical illustrates reactions involving different oxidation states of vanadium and asks you to explain them in terms of Eo values for the relevant half equations.
Given below are the colours of the different vanadium ions and some electrode potentials.
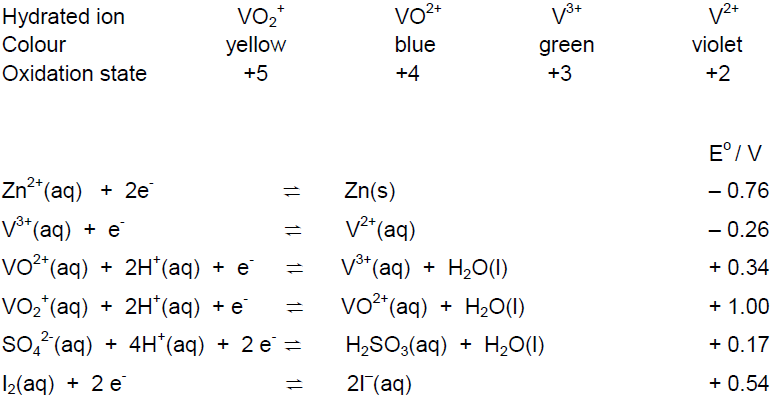
ENVIRONMENTAL CARE:
Solutions containing vanadium and zinc ions should be placed in the heavy metals container in the fume cupboard after use and not poured down the sink.
SAFETY:
Take care when adding the concentrated sulfuric acid. Safety glasses must be used and a lab coat must be worn. Do not add water to the concentrated acid.
PROCEDURE:
1. Preparation of standard solutions of vanadium(V) and vanadium(ll). Place about 0.25g of ammonium trioxovanadate(V) in a conical flask and add about 25 cm3 of dilute sulfuric acid. Carefully add about 5 cm3 of concentrated sulfuric acid and swirl the flask until you obtain a clear yellow solution. Pour about 2 cm3 of this vanadium(V) solution into each of two test tubes ready for later use. To the remainder in the conical flask add 1 - 2 g of zinc powder, a little at a time. Swirl the flask at intervals and record any changes taking place. When the solution has become violet (you may need to heat the flask for this final change) filter about 2 cm3 into each of three test tubes for your stock vanadium(ll) solutions.
2. Reactions of vanadium(V). To one of the tubes containing vanadium(V) add about 2 cm3 of potassium iodide solution and mix. Then add about 2 cm3 of sodium thiosulfate solution. Record your observations. To the second of the tubes containing vanadium(V) add a little sodium sulfite and shake. Filter if cloudy. Now boil carefully in a fume cupboard to remove excess SO2 and add one of the tubes of the vanadium(ll) solution. Record all your observations.
3. Reactions of vanadium(ll). To the second of the tubes containing vanadium(ll) add, a little at a time, an excess of potassium manganate(Vll) solution, shaking after each addition, until no further change is observed. Using the electrode potentials given above predict a way in which you could oxidize vanadium(ll) to vanadium(lll) and no further. Use the remaining tube of vanadium(ll) to test your prediction.
QUESTIONS.
1. Try to explain the above reactions using relevant electrode potentials and by writing balanced overall equations.
2. Why was sodium thiosulfate added in one of the reactions?
This worksheet can also be downloaded from:

 IB Docs (2) Team
IB Docs (2) Team