Environmental impact - heavy metals
 A.10 Environmental impact - heavy metals (3 hours)
A.10 Environmental impact - heavy metals (3 hours)
Pause for thought
Your students will need will to take some care when using solubility products.
Consider the following values (at 298 K) which are obtained from the table given in Section 32 of the data book[1].
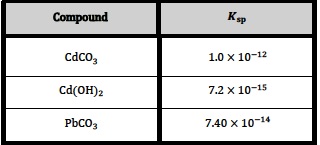
An obvious question might be; Which one of the above salts has the lowest solubility in water at 298 K?
Looking at the values for solubility products the answer might appear to be cadmium(II) hydroxide as its Ksp value is considerably lower than the Ksp values for the other two salts. In the 2001-2007 programme (last exams November 2008) students were expected to know that equilibrium constants had units and they could be asked to deduce them for particular reactions. Since then the IB has correctly stated that equilibrium constants have no units but this can lead to a little confusion when faced with a table of values such as the one given above. To compare the values meaningfully, students need to consider what equilibrium system each Ksp value is referring to.
Consider the first value of 1.0 x 10−12 for cadmium carbonate. This refers to:
CdCO3(s) ⇌ Cd2+(aq) + CO32−(aq)
and Ksp = [Cd2+(aq)] x [ CO32−(aq)]
For a saturated solution of cadmium(II) carbonate at 298 K, [Cd2+(aq)] = [ CO32−(aq)] = (1.0 x 10−12)½ = 1.0 x 10−6 mol dm−3
so the solubility of cadmium(II) carbonate at 298 K is 1.0 x 10−6 mol dm−3.
For cadmium(II) hydroxide, Ksp at 298 K is 7.2 x 10−15. The equilibrium system this value refers to is:
Cd(OH)2(s) ⇌ Cd2+(aq) + 2OH−(aq)
and Ksp = [Cd2+(aq)] x [OH−(aq)]2
For a saturated solution of cadmium(II) hydroxide [OH−(aq)] = 2 x [Cd2+(aq)]
so Ksp = 4 x [Cd2+(aq)]3, which means [Cd2+(aq)] = (7.2 x 10−15 ÷ 4)⅓ = (1.8 x 10−15)⅓ = 1.2 x 10−5 mol dm−3
The solubility of cadmium(II) hydroxide in water at 298 K is therefore 1.2 x 10−5 mol dm-3 which means that it is more soluble in water than cadmium(II) carbonate at 298 K.
However the Ksp value for lead(II) carbonate at 298 K is 7.40 x 10−14. By carrying out a similar calculation to the ones above it can be seen that the solubility of lead(II) carbonate in water at 298 K is 2.7 x 10−7 mol dm−3. Thus lead(II) carbonate is the least soluble of the three salts.
Although it would not affect the order for these three salts, note too that if solubilities are expressed in g dm−3 rather than mol dm−3 then this may also affect the order if the molar solubilities are close in value.
Nature of Science
Although scientific research often proceeds with perceived benefits in mind, there is a responsibility to also consider the risks and implications.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesConsider ethane−1,2−diamine to act as a bidentate ligand and EDTA4- to act as a hexadentate ligand. In biological processes the Haber–Weiss reaction naturally generates free radicals. Transition metals can catalyse the reaction. In the Fenton reaction the mechanism for generating reactive hydroxyl radicals involves the iron catalyst. Ksp values are given in Section 32 of the data booklet. International-mindednessConsider the responsibilities that scientists have for the global impact of their work. |
Teaching tipsThis is quite a difficult topic to teach but does provide plenty of opportunities for some interesting examination questions. Essentially you need to cover precipitation and chelation as two different ways of removing heavy metals from the environment and also mention adsorption. For precipitation you will need to cover the application of the equilibrium law to sparingly soluble (i.e. ‘insoluble’) salts. For binary salts the solubility product is equal to the product of the concentrations of the two ions at equilibrium but care needs to be taken with salts such as lead(II) hydroxide), Pb(OH)2, which form more ions in solutions (see “Pause for thought’ above). It is also worth mentioning how the common ion effect can increase the precipitation of metal ions by increasing the concentration of the anion in the saturated solution. Although it does not appear on the syllabus it might also be worth explaining how sometimes the common ion effect can lead to increased solubility due to complex ion formation. For example, zinc hydroxide redissolves in excess hydroxide ions to form [Zn(OH)4]2−(aq). Students already know how monodentate ligands use a non-bonding pair of electrons to form a coordinate bond to a transition metal ion. Explain that polydentate ligands contain more than one non-bonding pair of electrons so can have coordination numbers greater than 1. Use EDTA and ethane-1,2-diamine as examples and explain that as one ion of EDTA4− reacts with one ion of [Ni(H2O)6]2+, for example, the products are one ion of [Ni(EDTA)]2− and six molecules of water so the entropy change is positive. This means that ΔG is more negative (since ΔG = ΔH – TΔS) and the chelated complex will be stable as ΔG = −RTlnK . The Haber-Weiss reaction and the more specific Fenton reaction, which uses iron complexes to catalyse the formation of hydroxyl radicals, OH. from hydrogen peroxide and the superoxide free radical ion, O2−., need to be mentioned. The hydroxyl radical produced is very reactive and is used in various industrial processes. Adsorption can include the use of activated charcoal or clay etc. to remove heavy metal ions from polluted water. | Study guide
Pages 121 & 122 QuestionsFor ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Environmental impact - heavy metals. For short-answer questions see Environmental impact - heavy metals questions together with the worked answers on a separate page Environmental impact - heavy metals answers. Vocabulary listchelate/chelation |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A very simple explanation of Ksp using lead(II) iodide as an example by chemistNATE.
2. An animation of the common ion effect using silver iodide as an example.
3. A student demonstrates her Science Fair project on Fenton's reagent.
![]() Demonstration of Fenton's reagent.
Demonstration of Fenton's reagent.
Footnotes
- ^ Note that some of the Ksp values in the data booklet are given to two significant figures and some are given to three significant figures.

 IB Docs (2) Team
IB Docs (2) Team 





















