Condensation polymers questions
 Questions on condensation polymers
Questions on condensation polymers
1. Benzene−1,4−dicarboxylic acid, HOOC−C6H4−COOH, can react with propane−1,3−diol, HO−CH2−CH2−CH2−OH, to form a polymer.
(a) Identify the type of condensation polymer formed in this reaction.
(b) Draw the repeating unit of the polymer formed.
(c) State the balanced equation for the reaction.
(d) Calculate the atom economy of this reaction if 1000 mol of benzene−1,4−dicarboxylic acid reacts with 1000 mol of propane−1,3−diol to give a 100% yield of polymer.
2. The molecular formula of Kevlar is shown below.
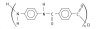
(a) Identify the class of condensation polymers that Kevlar belongs to.
(b) Deduce the structural formulas of the monomers used to produce Kevlar.
(c) Explain why a vest made from Kevlar is resistant to penetration by bullets.
(d) Explain why concentrated sulfuric acid considerably reduces the effectiveness of Kevlar as a protective material.
Download the Condensation polymers questions ![]() to give to your students.
to give to your students.
To see the worked answers go to Condensation polymers answers.

 IB Docs (2) Team
IB Docs (2) Team