MC test: Nuclear medicine
 Multiple choice test on D.8 Nuclear medicine
Multiple choice test on D.8 Nuclear medicine
Use the following 'quiz' to test your knowledge and understanding of this sub-topic. As this relates to a sub-topic on the options you may need access to the IB data booklet.
If you get an answer wrong, read through the explanation carefully to learn from your mistakes.
Which region of the electromagnetic spectrum is used to produce images for diagnostic purposes in magnetic resonance imaging, MRI?
MRI is short for nuclear magnetic resonance imaging and unlike CAT scans (computed tomography scans, which use X-rays) is non-invasive as it uses very low energy radio waves which is non-ionizing radiation.
In 2006 a former Russian intelligence officer, Alexander Litvinenko, was murdered when he drank a cup of tea containing polonium-210. Polonium-210 (Z = 84) decays naturally to lead-206 (Z = 82). What type of radiation caused his death by destroying his vital organs?
The mass number has decreased by 4 and the atomic number has decreased by 2 so an alpha particle is emitted during the breakdown of Po-210 to Pb-206.
The technetium isotope 99mTc is used as a diagnostic tool in hospitals. What does m stand for?
Tc-99m is an artificial metastable isotope of technetium. It emits gamma radiation and has a half-life of 6 hours. (It decays to 99Tc which is a weak beta emitter with a half-life of 2.1 x 105 years).
Which are known side effects of radiotherapy?
I. nausea
II. hair loss
III. fatigue
All three are common side effects of radiotherapy (although unlike chemotherapy, hair loss tends to be localised to the treatment area).
A pure sample of of iodine-131 was found to contain 3.125% of iodine-131 after 40 days. What is the half-life of iodine-131?
3.125% = 1/32. After five half-lives 1/32 of the original amount will be remaining. 40 days = 5 half-lives so the half-life = 8 days.
Bismuth-213 (Z = 83) has been used in targeted alpha therapy (TAT). It decays by both alpha and beta emissions. During beta emission it forms an isotope of polonium (Z = 84) which is also an alpha emitter. Other than alpha particles which initial product(s) will be formed from the alpha emissions of bismuth-213 and polonium-213?
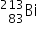 →
→ 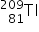 +
+ 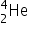 by alpha emission and
by alpha emission and 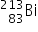 →
→ 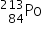 by beta emission which then decays to
by beta emission which then decays to 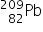 by alpha emission.
by alpha emission.
Which isotope is also formed in addition to alpha radiation during boron neutron capture therapy?
When non-radioactive boron-10 captures a neutron it breaks down to form an alpha particle and lithium-7, 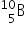 +
+ 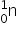 →
→ 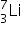 +
+ 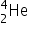 .
.
Which are correct representations of a positron?
I. 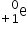
II. e+
III. β+
A positron is a positive electron so all three are correct
What type of reaction occurs during the preparation of technetium-99m from molybdenum-99?
Molybdenum has an atomic number of 42 and technetium has an atomic number of 43 so beta emission occurs as there is no change in mass but an increase of one in atomic number.
Tc-99m has a half-life of 6 hours. If a sample of pure Tc-99m is prepared at 0900 in the morning what percentage of Tc-99m will have decayed by 0900 on the next day?
24 hours is equal to four half-lives so 1/16 of the Tc-99m will remain. This means 15/16 of the sample, i.e. 93.75% (94% to two significant digits) will have decayed.

 IB Docs (2) Team
IB Docs (2) Team