Photovoltaic cells and DSSCs
 C.8 Photovoltaic cells and dye-sensitized solar cells (DSSCs) (3 hours)
C.8 Photovoltaic cells and dye-sensitized solar cells (DSSCs) (3 hours)
Pause for thought
Organometallic ruthenium compounds
A simple dye-sensitized solar cell is easy and cheap to make (see Practical work below) using a dye such as tea, onion skin or raspberries. Commercial cells are more expensive as they use platinum as the cathode although promising research has been carried out to replace the platinum with cobalt sulfide. This is much cheaper and also more efficient.
Commercial cells also use different light sensitive dyes. Some of the best dyes are organometallic complexes of ruthenium such as cis-bis(isothiocyanato)bis(2,2’-bipyridyl-4,4’-dicarboxylato ruthenium(II). These dyes are highly conjugated and absorb visible light efficiently over a wide wavelength range.
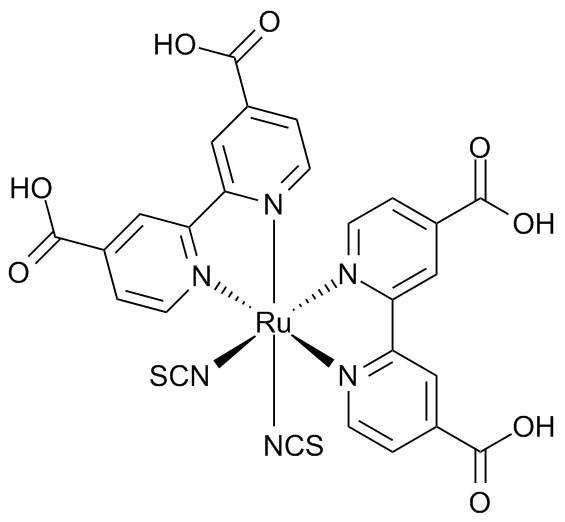
cis-bis(isothiocyanato)bis(2,2’-bipyridyl-4,4’-dicarboxylato ruthenium(II) used in DSSCs
Ruthenium has the ability to accommodate a wide range of oxidation states (from -2 to +8) and organometallic ruthenium compounds have many other uses. They are good catalysts for a wide variety of reactions, for example, dichlorotris(triphenylphosphine)ruthenium(II), RuCl2(PPh3)3, catalyses the oxidation of alcohols and phenols to ketones.
Organometallic ruthenium compounds are also used in chemotherapy and have fewer and less severe effects than platinum based drugs such as cis-platin, PtCl2(NH3)2. Organometallic ruthenium compounds are also showing promise as antimicrobial agents. This is an important area of research as many bacteria are now resistant to penicillins and there is a need to develop new classes of antimicrobials, rather than continue to synthesise drugs based upon analogues with known scaffolds.
Nature of science
The transdisciplinary nature of science can be illustrated by the operation of dye-sensitized solar cells as they use titanium dioxide nanoparticles to mimic photosynthesis.
The initial funding for the research and development of the first photovoltaic cells came from NASA who employed them in space probes.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesRelate the relative conductivity of metals and semiconductors to ionization energies. Only a simple treatment of the operation of the cells is required. Introducing a small amount of a group 13 element in p-type semiconductors creates electron holes in the crystal. The inclusion of a group 15 element in n-type semiconductors provides extra electrons. In photovoltaic cells the light is absorbed and the charges separated in the silicon semiconductor. In dye-sensitized solar cells the processes of absorption and charge separation are separated. Specific redox and electrode reactions taking place in the newer Grätzel DSSC should be covered. An example is the reduction of I2/I3─ ions to I─. International-mindednessCountries with good supplies of sunlight and unused land have the potential to improve economically by harnessing solar energy efficiently. |
Teaching tipsThis is a mainly a factual sub-topic based more on explanation and memory as there are no quantitative aspects to it. Explain that organic molecules containing a pi bond absorb light in the ultraviolet region as an electron is excited to a pi* anti-bonding orbital. As the amount of conjugation (delocalization) increases the pi* anti-bonding orbital becomes lower in energy so light of longer wavelength is absorbed. The dyes used in DSCCs contain extensive conjugation so absorb light in the visible region. The photoelectric effect and the doping of silicon to increase the conductivity are well known. Dye-sensitized solar cells have been around since 1972 although the low cost Grätzel cell was not invented until 1988. Students will need to understand the function of each component of a Grätzel cell and be able to write equations for the redox reactions that occur between iodide and triiodide ions in the electrolyte. Because they are low cost you might wish to experiment with a DSSC as part of your non-mandatory practical programme (see video below and the article by J.Twiddle and others published in the School Science Review). Stress the main operating difference between DSSCs and silicon based photoelectric cells. In the DSCCs the production of the photoelectrons by absorption of light is separate to the separation of the charges leading to the current produced (which is why it is said to mimic the photoelectric effect) whereas in silicon cells these two process occur together in the silicon semiconductor. It is worth getting your students to challenge the statement in the clarification notes as to whether the conductivity of metals is related to ionization energies. Firstly ionization energies refer to the gaseous state whereas conductivity normally refers to the solid state . Secondly silver (1st ionization energy 731 kJ mol-1), which is the best conducting of all metals, has a higher first ionization energy than many metals that are much poorer conductors, e.g lead (1st ionization energy 716 kJ mol-1). | Study Guide:Page 152 QuestionsFor ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Photovoltaic cells & DSSCs. For short-answer questions see Photovoltaic cells and DSSCs questions together with the worked answers on a separate page Photovoltaic cells and DSSCs answers. Vocabulary listphotoelectric effect Practical work |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A good explanation of how photovoltaic cells work including n- and p- doping by Thomas Schwenke.
![]() Solar energy & photovoltaic cells
Solar energy & photovoltaic cells
2. An animation showing how dye-sensitized solar cells are constructed and work.
3. Michael Grätzel talks about his own dye-sensitized solar cell.

 IB Docs (2) Team
IB Docs (2) Team 













