Nuclear medicine
 D.8 Nuclear medicine (4 hours)
D.8 Nuclear medicine (4 hours)
Pause for thought
Technetium-99m
Although much of this sub-topic deals with cancer treatment, technetium-99m, the most widely used radioisotope in medicine, is only used in diagnostic radiotherapy. It is ideal for this purpose as it is a gamma radiation emitter with a half-life of 6.0 hours. This means that within 24 hours 94% of it will have decayed to technetium-99. It also has a short biological half-life of one day, which means that it is readily excreted in urine from the body. Several other factors also make it an ideal diagnostic tool. The radiation emitted can easily be followed by conventional X-ray equipment and the breakdown product, technetium-99, has a much longer half-life of 2.11 x 105 years. Technetium-99 is a beta emitter, which breaks down to form stable ruthenium-99, this also contributes to keeping the patient’s total exposure to radiation low – although it is not totally risk free.
Technetium-99m is an example of a nuclear isomer. Technically a nuclear isomer is caused by the excitation of one or more of the nucleons (protons or neutrons) of an atomic nucleus to give a metastable state. It is metastable compared to the normal excited nuclear state as it has a longer half-life as it reverts back to the ground state. Even so the half-life of a nuclear isomer is normally very short (greater than 1.0 x 10-9 s) but some metastable isomers, including technetium-99m have longer half-lives measurable in minutes or hours.
Although technetium-99m’s half-life of 6 hours makes it ideal for diagnostic treatment it does pose a problem of storage, as the hospital needs a fresh supply every day that it is going to be used. In fact, hospitals are supplied with its parent nucleus, molybdenum-99, and they use technetium-99m generators to prepare the metastable isotope through the beta-decay of molybdenum-99.
The isotope is chemically extracted using column chromatography usually as the water-soluble sodium salt of the technetate(VII) ion, TcO4−. It is then normally reduced to the +4 or +3 oxidation state and converted in a complex ion using a ligand, such as exametazine, for diagnostic use.
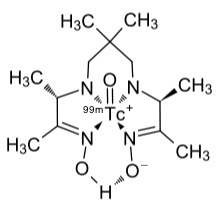
Tc-99m exametazine complex
Nature of science
The benefits of the technique(s) being considered need to be balanced against the risks associated with the exposure to radiation.
Learning outcomesAfter studying this topic students should be able to: Understand
Apply their knowledge to:
| Clarification notesThe discussion of common side effects should include loss of hair, nausea, fatigue and sterility. International-mindednessCulture, cost, availability and beliefs are some of the factors that can influence the use of nuclear technology in medicine and explain why its use is not consistent throughout the world. |
Teaching tipsAlthough the use of radioisotopes in medicine is mentioned briefly under ‘utilization’ in sub-topic 2.1 : The nuclear atom, this is really the first time students will have encountered radioactivity. Explain to them the different types of radiation and make sure they know how to write the symbols for an electron (β- particle), proton, neutron, alpha particle and a positron (β+ particle) and then give them plenty of practice at writing nuclear equations, taking care to include all the isotopes listed in the guide, i.e. Tc-99m, Lu-177, Y-90, I-131 and Pb-212. They should also be familiar with the formation of Tc-99m (from Mo-99) and its subsequent breakdown to Tc-99 and Ru-99 and the reaction of boron-10 with a neutron. Make sure they fully understand the concept of half-life and give them plenty of practice with half-life calculations. The data booklet is quite confusing here. There are three different equations listed in Section 1. Probably the easiest (where it does not involve a whole number of half-lives, which can be done by inspection) is to calculate λ by dividing 0.693 (ln 2) by t½ then use the equation N = Noe−λt. The rest of the topic is mainly learning about why technetium-99m is used in diagnostic medicine, common side effects of radiotherapy, how TAT and BNCT work and the application and non-invasiveness of NMR (which changes its name to MRI in medicine so that patients are not alarmed by the use of the word ‘nuclear’). You could ask students to prepare short presentations on each of these areas. | Study GuidePage 163 QuestionsFor ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Nuclear medicine For short-answer questions see Nuclear medicine questions together with the worked answers on a separate page Nuclear medicine answers. Vocabulary listpositron |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. Students already know about 1H NMR from Topics 11 and 21. This video by, Howard R. Hart Jr., one of the pioneers of MRI, explains how an MRI scan works.
2. A concise animated video by Richard Thornley on TAT, targeted alpha therapy.
3. A really neat video, also by Richard Thornley, which clearly explains BNCT, boron neutron capture therapy.

 IB Docs (2) Team
IB Docs (2) Team 





















