Topics 10 & 20
Including International-mindedness, TOK, and 'Utilization' in Organic Chemistry
International-mindedness
![]()
![]() 1. Hazard symbols
1. Hazard symbols
 As many goods are transported around the world it obviously makes sense that there are international agreements on the use of Hazard Symbols for potentially dangerous chemicals (many, but by no means all, of these chemicals are organic). Some of these agreements are truly international and some are slightly more localized such as the European Agreement concerning the International Carriage of Dangerous Goods by Road. Since our students are heavily engaged in practical work they should know and understand the universally recognised symbols for the different types of hazards associated with chemical substances. There are several sites where you can test your knowledge of hazard symbols.
As many goods are transported around the world it obviously makes sense that there are international agreements on the use of Hazard Symbols for potentially dangerous chemicals (many, but by no means all, of these chemicals are organic). Some of these agreements are truly international and some are slightly more localized such as the European Agreement concerning the International Carriage of Dangerous Goods by Road. Since our students are heavily engaged in practical work they should know and understand the universally recognised symbols for the different types of hazards associated with chemical substances. There are several sites where you can test your knowledge of hazard symbols.
![]()
![]() 2. An international ban on BCF fire extinguishers
2. An international ban on BCF fire extinguishers
.JPG)
Theory of Knowledge
Organic Chemistry obviously lends itself to many different connections to TOK. The core part of the syllabus only has two specific links. Sub-topic 10.1 Fundamentals of organic chemistry talks about the use (or misuse) of labels. The origin of the label 'Organic chemistry' was based on the misconception that some vital force was needed to explain the chemistry of carbon compounds. The syllabus questions whether there are other similar examples elsewhere and whether the label should be changed once the truth of the matter is recognised. The second example referred to in the same sub-topic is based on Kekulé's claim that his explanation for the cyclic structure of benzene emanated from a dream. It questions the role played by less analytical ways of knowing in the acquisition of scientific knowledge.
One interesting aspect of TOK and the Nature of Science is the value of models. This is mentioned specifically in the AHL sub-topic 20.3 Stereoisomerism particularly ways of representing three-dimensions using two-dimensional diagrams. I have written about modelling in the page on Computer modelling under ICT in practical work but this is more for computer simulations. It is certainly worth looking at modelling in chemistry in more depth and this is covered below in Example 1 and, separately in the Key terms & concepts of NOS. Sub-topic 20.3 also refers to optical isomerism and links it to the indirect evidence that enantiomers provide to confirm the existence of tetrahedrally-bonded carbon atoms. The point being that the power of reasoning allows chemists to gain access to the molecular scale. This is illustrated well by Walden inversion during SN2 reactions and I have included it below as Example 2.The role of language is also important, particularly the use of the IUPAC system of naming organic compounds. This is a tool which enables chemists to clarify and distinguish between different organic compounds and their structures. I have written a separate page on the Language of Chemistry in the TOK section on this site and also linked this to a page specifically on IUPAC.
There is a further reference to TOK in the Additional Higher Level material under sub-topic 20.2 which questions the roles of imagination, intuition and reasoning in finding solutions to practical problems.
![]()
![]() 1. Modelling organic molecules
1. Modelling organic molecules
 When you ask students to reflect on the types of models they have come across they may have encountered some more abstract forms of modelling in subjects like Economic but most will think of dolls, model aeroplanes, model boats etc. etc. From their childhood they will think of models as being smaller than the objects they represent. In chemistry the opposite is true. The models we have of molecules are many times bigger than the molecules they represent. Think of the word ‘methane’. It will sum up many images and much information and in a sense the word methane is itself a model. Certainly just writing methane does not mean we have methane itself magically on the page. The surrealist painter René Magritte realised this in his famous painting ‘Ceci n'est pas une pipe’.
When you ask students to reflect on the types of models they have come across they may have encountered some more abstract forms of modelling in subjects like Economic but most will think of dolls, model aeroplanes, model boats etc. etc. From their childhood they will think of models as being smaller than the objects they represent. In chemistry the opposite is true. The models we have of molecules are many times bigger than the molecules they represent. Think of the word ‘methane’. It will sum up many images and much information and in a sense the word methane is itself a model. Certainly just writing methane does not mean we have methane itself magically on the page. The surrealist painter René Magritte realised this in his famous painting ‘Ceci n'est pas une pipe’.
There are many different models of methane that are commonly used. Some of them are:
.jpg)
Five of those given above will be familiar to Standard and Higher Level students and the sixth (image 5) to Higher Level students only. You could ask students what information about methane is carried in each of the six pictures because they do all represent a different aspect of methane’s attributes. Students will also realise that they make assumptions themselves about the models and what they represent. For example, the 90o angles in the second model are known to represent 109o 28’ but are drawn as 90o for ease of drawing in two dimensions etc. Which is the true model? Model number 5 is perhaps the most sophisticated as it tries to convey the concept of sp3 hybridization and shape but it is only one small step further towards representing methane. Of course in reality none of them is the real methane. Each gives some specific information about methane but we are limited by the constraints of dimension and size (to name just two of the many constraints) to give anything like a true representation of a methane molecule.
![]() 2. Walden inversion
2. Walden inversion
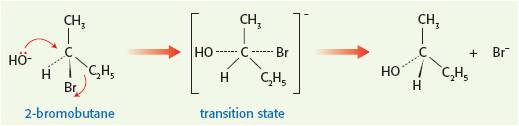
As you can see from the TOK aspects of Topics 6 & 16 it is possible to disprove a mechanism from the experimentally determined rate equation but it is not possible to prove it. You can only say that the proposed mechanism is consistent with the rate equation. A Latvian chemist, Paul Walden (1863 - 1957), applied fantastic logic to 'prove' the SN2 mechanism for certain nucleophilic substitution reactions and in the process also confirmed the tetrahedral structure of halogenoalkanes. He reasoned that if the initial halogenoalkane was optically active then if the mechanism went through an SN1 type mechanism then the product would be a racemic mixture of the two enantiomeric alcohols as the hydroxide ion could attach to either side of the carbocation intermediate in a 50:50 ratio. However if it went by an SN2 type mechanism, via a five-membered transition state, the resulting alcohol would rotate the plane of plane-polarized light in the opposite direction to the starting material. The product did indeed rotate the plane of plane-polarized light in the opposite direction to the reactant.
'Utilization'
There are many links to 'Utilization' in Topics 10 & 20. For example, the use of alkanes as fuels, alcohols as fuel additives and alkenes in the ripening of fruit. Esters have a variety of uses, for example, for food flavourings, perfumes and solvents. Another major utilization of organic compounds is the whole of the plastics industry. When the price of crude oil changes it not only affects the cost of fossil fuels but also a huge range of other materials such as plastics and drugs for which crude oil acts as the feedstock.
![]()
![]() 1. Alcohol abuse and society
1. Alcohol abuse and society
 Sub-topic 10.2 Alcohols includes some of the basic chemistry of alcohols. Alcohol in chemistry has a different meaning to ‘alcohol’ in society where the term is used to refer to just one particular alcohol – ethanol. Students are often given information and advice about the dangers of taking drugs and yet often alcohol is not included in the list of drugs. In many parts of the world the most dangerous drug taken both in terms of cost in lives (other people’s lives as well as the drinker’s life in traffic accidents for example) and in cost in crime and money is ethanol. This is a cultural problem which affects some countries worse than others. When teaching the chemistry of alcohols it is worth mentioning the dangers associated with imbibing too much alcohol. This can easily be related to the breathalyser test involving acidified dichromate(VI) ions when covering the oxidation of alcohols (10.2). Binge drinking is now a common occurrence in many town centres. The number of young women with serious liver failure problems due to drinking has increased considerably during the past two decades. If drinking alcohol is part of the culture of your society then impress upon your students that they need to learn to drink in moderation.
Sub-topic 10.2 Alcohols includes some of the basic chemistry of alcohols. Alcohol in chemistry has a different meaning to ‘alcohol’ in society where the term is used to refer to just one particular alcohol – ethanol. Students are often given information and advice about the dangers of taking drugs and yet often alcohol is not included in the list of drugs. In many parts of the world the most dangerous drug taken both in terms of cost in lives (other people’s lives as well as the drinker’s life in traffic accidents for example) and in cost in crime and money is ethanol. This is a cultural problem which affects some countries worse than others. When teaching the chemistry of alcohols it is worth mentioning the dangers associated with imbibing too much alcohol. This can easily be related to the breathalyser test involving acidified dichromate(VI) ions when covering the oxidation of alcohols (10.2). Binge drinking is now a common occurrence in many town centres. The number of young women with serious liver failure problems due to drinking has increased considerably during the past two decades. If drinking alcohol is part of the culture of your society then impress upon your students that they need to learn to drink in moderation.
![]()
![]() 2. Plastics
2. Plastics
There is so much written on plastics that it is difficult to know where to begin. Since addition polymers are specifically mentioned in the core (sub-topic 10.2 Alkanes, alkenes & addition polymers) and condensation reactions too (but not condensation polymers) it makes sense to at least mention some of the connections with ‘Utilization’ as you teach them. These include social (ask your students to make a list of all the plastic materials they come into contact with on a daily basis), and environmental (how do you dispose of plastics?).

 IB Docs (2) Team
IB Docs (2) Team