 Assignment:
Assignment:  Questions on Topic 20.1: Nucleophilic substitution
Questions on Topic 20.1: Nucleophilic substitution
This page of questions can be marked as direct student access either for assigning as a test or for students to work on in their own time. If you do not wish to use student access, links to downloadable versions of the questions and, separately the worked answers, can be found at Printable versions of written tasks.
Explain the following statements:
i. hydroxide ions are better nucleophiles than water molecules.
3 lines
Both hydroxide ions and water molecules possess non-bonding pairs of electrons but the density of negative charge will be much higher on the ion than on the neutral water molecule. The hydroxide ion will therefore have a greater attraction towards the partially positive carbon atom bonded to the electronegative halogen atom in the halogenoalkanes.
ii. the hydrolysis of iodoethane is faster than the hydrolysis of bromoethane.
2 lines
The carbon to iodine bond in iodoethane is much weaker than the carbon to bromine bond in bromoethane so is more easily broken.
iii. the hydrolysis of 2-bromo-2-methylpropane is faster than the hydrolysis of 1-bromobutane.
5 lines
2-bromo-2-methylpropane is a tertiary halogenoalkane. The positive inductive effect of the three methyl groups helps in the formation of an intermediate carbocation so the mechanism is SN1. The activation energy required to form this carbocation is less than the activation energy required to form the transition state in the SN2 mechanism for the hydrolysis of the primary halogenoalkane (1-bromobutane).
iv. fluoroethane does not react with dilute sodium hydroxide solution to form ethanol.
1 line
The carbon to fluorine bond is too strong to be broken.
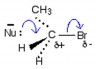
Explain the mechanism for the reaction of ammonia with bromoethane. (Use curly arrows to show the movement of pairs of electrons and upload your answer as an image or a pdf file.)
5 lines
 Note that the reactions of nucleophiles other than the hydroxide ion do not appear to be on the syllabus, so this reaction does not need to be learned. In fact the final product formed will be the substituted ammonia salt, (C2H5)NH3+Br− as an acid and a base are present.
Note that the reactions of nucleophiles other than the hydroxide ion do not appear to be on the syllabus, so this reaction does not need to be learned. In fact the final product formed will be the substituted ammonia salt, (C2H5)NH3+Br− as an acid and a base are present.
i. Explain why the substitution of primary halogenoalkanes by hydroxide ions could also be classed as a Lewis acid-base reaction.
2 lines
With primary halogenoalkanes the hydroxide ion is donating a pair of electrons to the carbon atom (which is accepting a pair of electrons) when it forms the transition state.
ii. Explain whether the reaction of tertiary halogenoalkanes with hydroxide ions could also be classed as a Lewis acid-base reaction.
3 lines
The second step in the SN1 mechanism with tertiary halogenoalkanes can also be considered a Lewis acid-base reaction as the intermediate carbocation behaves as a Lewis acid when it accepts a pair of electrons from the hydroxide ion (which is behaving as a Lewis base).
Suggest how tetramethylammonium bromide, (CH3)4N+Br–, could be made from bromomethane.
2 lines
React a large excess of bromomethane with ammonia. (Successive nucleophilic substitution reactions will occur and the product will be formed via methylamine, CH3NH2, dimethylamine(CH3)2NH, and trimethylamine, (CH3)3N.)
 Assignment:
Assignment: ![]() Questions on Topic 20.1: Nucleophilic substitution
Questions on Topic 20.1: Nucleophilic substitution 
 IB Docs (2) Team
IB Docs (2) Team 