Electrochemical cells questions
 Assignment:
Assignment: 
 Questions on Topic 9.2 : Electrochemical cells questions
Questions on Topic 9.2 : Electrochemical cells questions
This page of questions can be marked as direct student access either for assigning as a test or for students to work on in their own time. If you do not wish to use student access, links to downloadable versions of the questions and, separately the worked answers, can be found at Printable versions of written tasks.
A student carried out an experiment to determine the cell voltage (electromotive force) of several different simple voltaic cells. She obtained the following results:

i. Deduce the cell voltage she would have obtained if she had connected the magnesium half-cell to the iron half-cell.
ii. Identify the positive (cathode) and negative (anode) electrode when a zinc half-cell is connected to an iron half-cell and state the half-equations and the overall equation when the cell is operating.
Explain why sodium in both the solid and molten form is a good conductor of electricity whereas sodium in the gaseous state is a poor conductor of electricity.
i. Explain why solid sodium chloride is a poor conductor of electricity whereas molten sodium chloride is a good conductor of electricity.
ii. Sodium chloride is easily available as it can be bought as ‘table salt’. Explain why it is difficult to perform an experiment in a school laboratory to determine whether or not it conducts electricity when molten.
i. Deduce the products formed at the positive and negative electrodes when electricity is passed through molten magnesium chloride and give the half-equations for the reactions occurring.
ii. During the electrolysis of molten magnesium chloride the current passes from the battery through the wires to the electrodes then through the molten electrolyte. Explain the different ways in which electricity is conducted in (I) the wires and (II) the electrolyte.
Iron is above copper in the reactivity series.
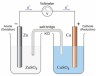
i. Draw a diagram of a voltaic cell made up from an iron half-cell and a copper half-cell and upload it as an image or s a pdf file.
On your diagram clearly label:
- the positive electrode (cathode)
- the negative electrode (anode)
- the direction of flow of electrons in the external circuit when the cell is operating.
ii. Give the half- equations for the reactions occurring at each electrode when the cell is operating and deduce the overall equation for the reaction.
i. Draw a labelled diagram to show the electrolysis of molten lead(II) iodide, PbI2(l). (Upload your answer as an image or as a pdf file.)
ii. State the half-equation for the reaction that occurs at the positive electrode (anode) and name the product.
iii. Identify whether the reaction taking place at the positive electrode (anode) is oxidation or reduction.
iv. State the half-equation for the reaction that occurs at the negative electrode (cathode) and name the product.
v. Identify whether the reaction taking place at the negative electrode (cathode) is oxidation or reduction.

 IB Docs (2) Team
IB Docs (2) Team 
