MC test: The nuclear atom

 Multiple choice test on 2.1 The nuclear atom
Multiple choice test on 2.1 The nuclear atom
Use the following 'quiz' to test your knowledge and understanding of this sub-topic. You will need access to a periodic table (Section 6 of the IB data booklet). You may find a calculator useful to answer some of the questions.
If you get an answer wrong, read through the explanation carefully to learn from your mistakes.
Which is a correct statement about the relative mass and charge of a sub-atomic particle?
The relative masses and charges for protons, neutrons and electrons are 1, +1; 1, 0 and 0, −1 respectively.
Which are correct statements about an atom?
I. All atoms are made up of protons, neutrons & electrons.
II. Protons & neutrons are located in the nucleus of a atom.
III. Virtually all of the mass of an atom is concentrated in its nucleus.
Although almost all atoms are made up of protons, neutrons and electrons, an atom of hydrogen, 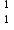 H, contains no neutrons. so the statement "All atoms are made up of protons, neutrons & electrons" is not correct.
H, contains no neutrons. so the statement "All atoms are made up of protons, neutrons & electrons" is not correct.
Which statement is correct about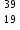 K+?
K+?
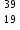 K+ contains 19 protons, 20 neutrons and 18 electrons.
K+ contains 19 protons, 20 neutrons and 18 electrons.
Which are correct statements about the 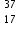 Cl− ion?
Cl− ion?
I. The atomic number of chlorine is 37.
II. The ion contains 18 electrons.
III. The ion contains 20 neutrons.
The atomic number is the subscript on the left, in this case 17, not 37 so I is wrong.
What are X, Y and Z when the table is completed?
| Symbol | Number of protons | Number of neutrons | Number of electrons | Atomic number | Mass number |
| X | Y | 28 | 21 | 24 | Z |
The mass number, A, equals the number of protons and neutrons and is the superscript on the left, the atomic number, Z, is the subscript on the left. It is a 3+ ion as there are three less electrons than protons.
Boron has a relative atomic mass of 10.81. There are two stable isotopes of boron, boron-10 and boron-11. What is the abundance of boron-10 in naturally occurring boron?
Let there be x% of boron-10 and y% of boron-11.
10x + 11y = 10.81 x 100 and x + y = 100
10x + 11(100 − x) = 1081; so x = 19 and y = 81
The mass spectrum of an element shows two peaks at 191 and 193 with relative abundances of 38.5% and 61.5% respectively. What is the relative atomic mass of the element?
Ar= [(38.5 x 191) + (61.5 x 193)] ÷ 100 = 192.23
The graph show the mass spectrum of strontium.

What is the relative atomic mass of strontium according to this graph?
Ar= [(0.56 x 84) + (9.9 x 86) + (7.0 x 87) + (82.6 x 88)] ÷ 100 = 87.76
Which species contains 10 neutrons and 8 electrons?
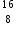 O contains only 8 neutrons. The remaining three answers all contain 10 neutrons but F− and Ne both also have 10 electrons whereas F+ has only eight electrons.
O contains only 8 neutrons. The remaining three answers all contain 10 neutrons but F− and Ne both also have 10 electrons whereas F+ has only eight electrons.
Which statements about different isotopes of the same element are correct?
I. They have almost identical chemical properties.
II. They have different physical properties.
III. They contain a different number of neutrons in their nucleus.
Isotopes of the same element have the same number and arrangement of electrons so their chemical properties are virtually identical but may differ very slightly (e.g. their rate of reaction), particularly with the lighter elements such as hydrogen. Their physical properties (e.g. melting point) are different as they have different masses.

 IB Docs (2) Team
IB Docs (2) Team