The nuclear atom questions
 Assignment:
Assignment: 
 Questions on Topic 2: The nuclear atom
Questions on Topic 2: The nuclear atom
This page of questions can be marked as direct student access either for assigning as a test or for students to work on in their own time. If you do not wish to use student access, links to downloadable versions of the questions and, separately the worked answers, can be found at Printable versions of written tasks.
Working sequentially from left to right identify all the missing components in each row to complete the following table.
.png)
Two isotopes used in medicine are 125I and 131I. The atomic number of iodine is 53.
i. State how they would differ in:
(a) the number of neutrons contained in the nucleus of each isotope.
(b) their physical properties
(c) their chemical properties
ii. Suggest why people living near the nuclear power plant in Fukushima, which was damaged in the earthquake that hit Japan in March 2011, were given iodine tablets.
A naturally occurring sample of chromium contains the following relative amounts of four different isotopes.

Determine the relative atomic mass of chromium to two decimal places.
The relative atomic mass of bromine is 79.91. Determine the relative percentage abundance of its two isotopes 79Br and 81Br to two decimal places.

 IB Docs (2) Team
IB Docs (2) Team 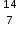 N, 7,14
N, 7,14 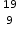 F−, 9,19,9
F−, 9,19,9 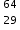 Cu2+, 29,35
Cu2+, 29,35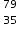 Br−, 35,36,35,79
Br−, 35,36,35,79.png)